Google Scholar | Pubmed | Biography
Unravelling the role of cell signalling in our molecular mechanistic understanding of amyotrophic lateral sclerosis/motor neuron disease
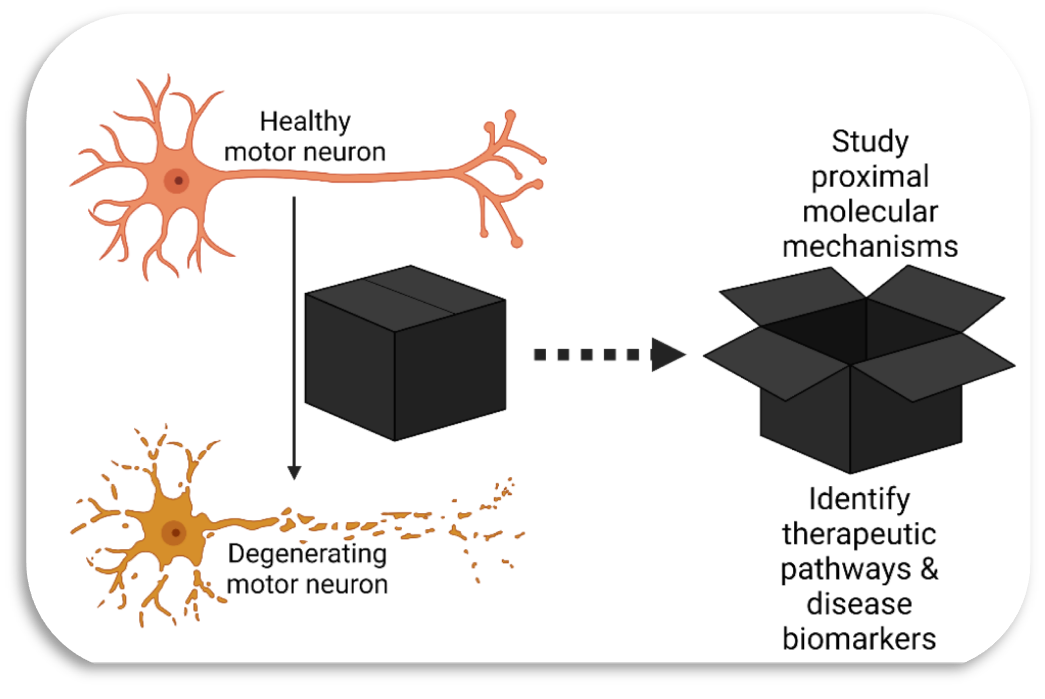
Our lab studies cell signalling mechanisms in amyotrophic lateral sclerosis (ALS), a devastating neurological disorder, characterised by the degeneration of motor neurons, without an effective disease-modifying treatment. Major advances in genetics have uncovered a plethora of ALS variants in genes linked to cell signalling, for example, NEK1 and TBK1. In the ALS field, there has been insufficient dedicated research on kinases, despite selective motor neuron degeneration being linked to the aberrant regulation of kinases. Inhibition of the Src/c-Abl pathway in multiple models of ALS (and via screening based on a survival assay of human stem cell-derived motor neurons from people with ALS) suggests that kinases closely regulate shared downstream processes in ALS pathogenesis. Moreover, aberrant phosphorylation of various ALS-related proteins (e.g., TDP-43 [known to be phosphorylated by CK1δ] and FUS) by kinases could affect their cellular localisation and, consequently, their biological function.
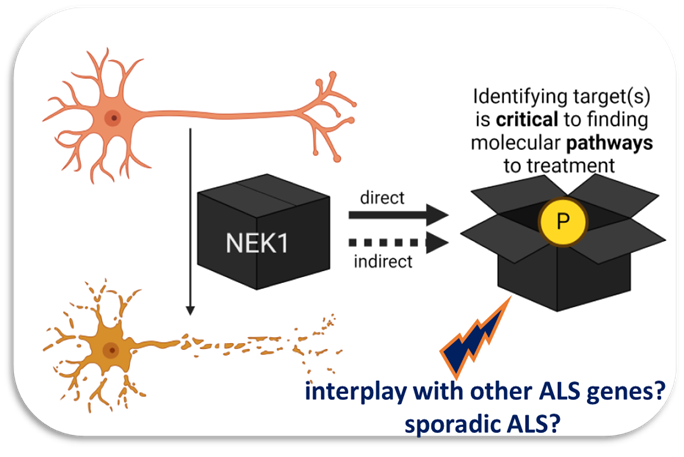
Arpan plans to decipher the biology in human motor neurons that is impacted by the NEK1 protein kinase, which is one of the commoner mutations underlying ALS. He plans to undertake experiments to define the key physiological phosphorylation targets of NEK1 in motor neurons and understand the function that phosphorylation of these targets plays. He will also undertake translational studies to investigate whether defective phosphorylation of NEK1 targets is observed in sporadic ALS patient biosamples and whether these targets can be employed as future biomarkers to stratify NEK1-driven ALS. Overall, Arpan’s ambition as a practising clinical neurologist is to make fundamental discoveries leading to transformative knowledge and the development of key reagents that will help advance our understanding of ALS—its diagnosis and treatment—in the future.
Arpan would be delighted to hear from anyone interested in joining his laboratory, situated in a Unit where all PIs share an ethos that prioritises personal wellbeing and career development, and a positive research culture. Informal enquiries are welcomed via email.
Collaborators
Professor Kevin Talbot, Head of Department & Professor of Motor Neuron Biology, University of Oxford.
Professor John Rouse, Professor of Chromosome Biology, MRC PPU, University of Dundee.
Professor Colin Smith, Director of Centre for Clinical Brain Sciences & Professor of Neuropathology, Euan MacDonald Centre for MND Research, University of Edinburgh.
Dr Wenting Guo, Principal Investigator, NeuroStra Institute, Strasbourg, France.
Dr Raja Nirujogi, Independent Investigator, MRC PPU, University of Dundee.


