Embryonic Stem Cells (ESCs) have the potential to provide tissue replacement therapies for a number of debilitating diseases. This is because ESCs have the capacity to differentiate into any cell type in the adult body, a property known as pluripotency.
A major research effort is to identify new signaling pathways which control ESC pluripotency and differentiation. To tackle this problem, members of the Findlay lab teamed up with Nathanael Gray’s lab at Harvard Medical School.
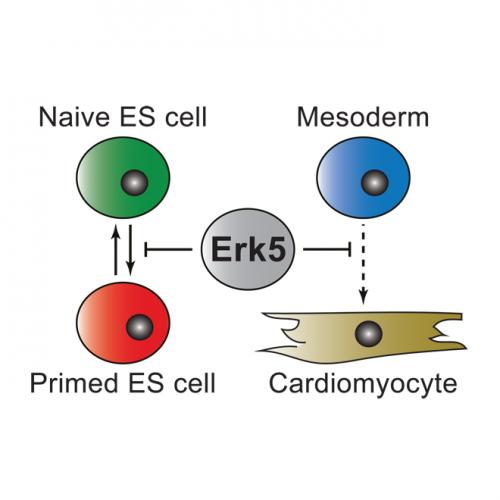
In a high-throughput screen of selective small molecule kinase inhibitors, Charlie Williams, a PhD student in the Findlay lab, discovered that ERK5 inhibitors promote transition of so-called naïve ESCs into the primed state, where they acquire the ability to differentiate.
Charlie Williams and the team used an elegant combination of chemical engineering and genome editing strategies to confirm that the ERK5 signaling pathway maintains ESCs in a state of naïve pluripotency. Rosalia Fernandez-Alonso, a postdoctoral fellow in the Findlay lab, then showed that ERK5 specifically regulates differentiation of ESCs to cardiac muscle cells, or cardiomyocytes.
Greg Findlay, who led the research, said “Our findings have significant implications for tissue regeneration. Most excitingly, our results suggest that Erk5 inhibitors may instruct ESCs to form cardiac cells, which can be exploited to help repair damaged tissue following a heart attack.” In future, the lab will explore this possibility and investigate the mechanisms by which Erk5 controls ESC pluripotency and differentiation.
Professor Dario Alessi, Director of the MRC unit stated, “Congratulations to Greg, Charlie and Rosalia for this very important paper that provides significant new insights into the role that the ERK5 signaling pathway plays in biology. The ERK5 pathway has been a bit of a mystery at least for me, and this study, which is also the first publication from the Findlay laboratory, defines a clear-cut important role for the ERK5 network in controlling differentiation of stem cells. It has ramifications for how differentiated stem cell-derived lineages such as cardiomyocytes might be better produced in the future. I am also excited about the area that this opens up to better understand the molecular mechanism by which ERK5 controls these pathways in future research”.
To read Charlie and Rosalia’s paper, which is published in Cell Reports today, click here.

