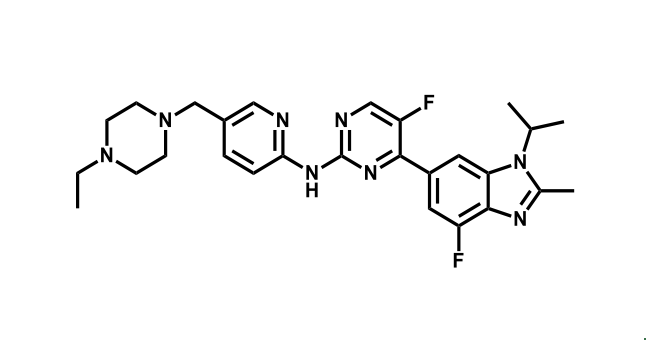
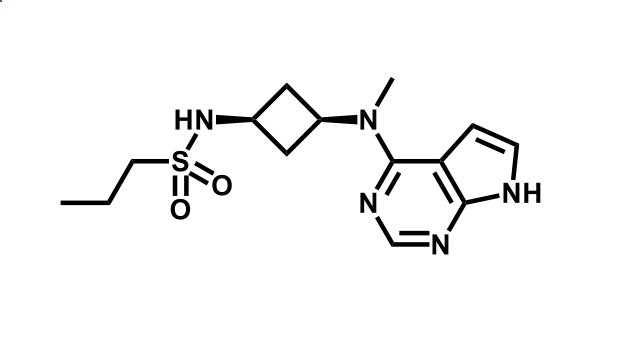
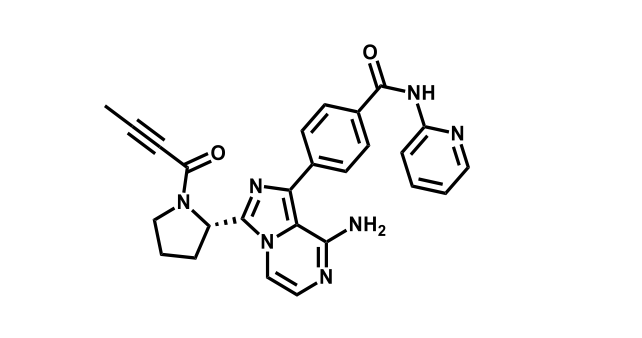
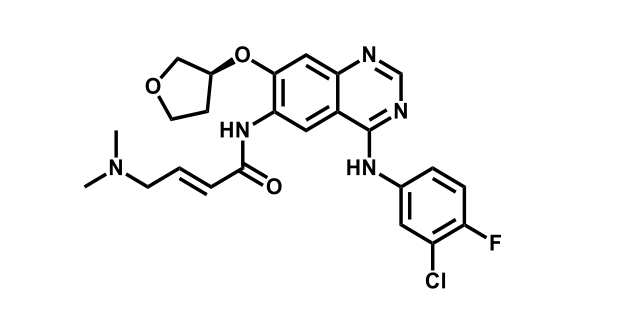
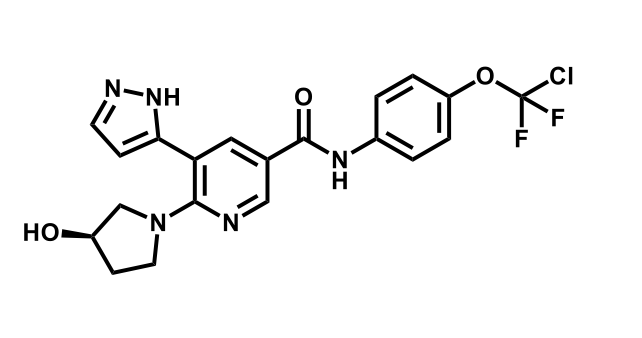
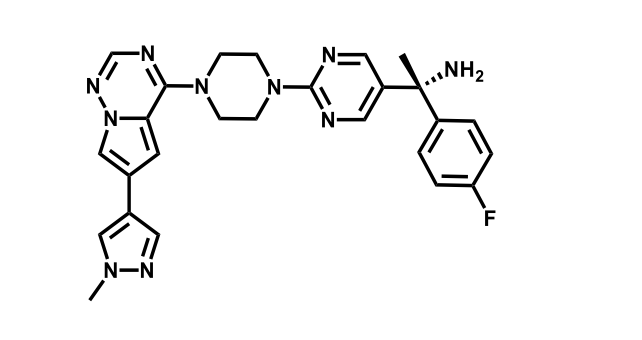
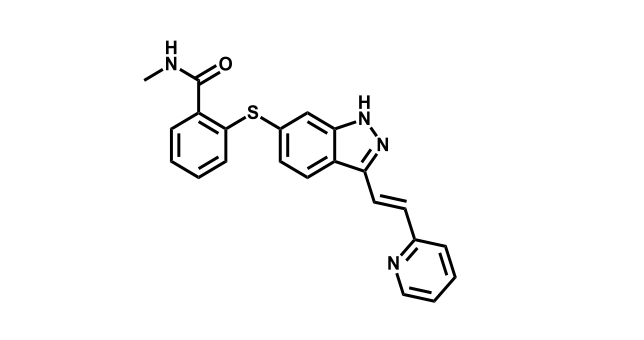
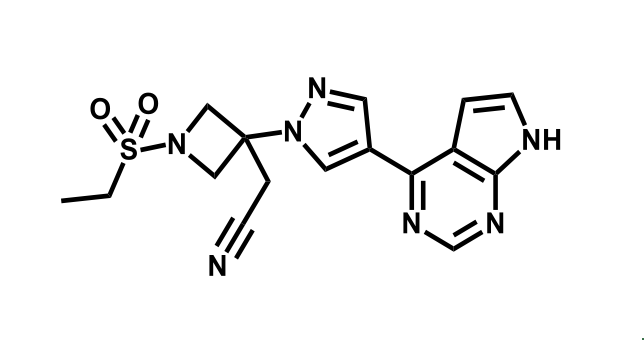
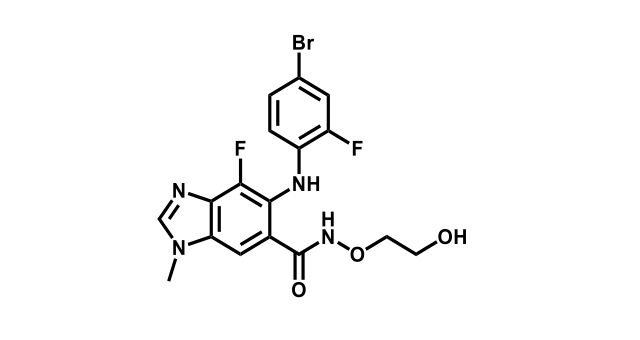
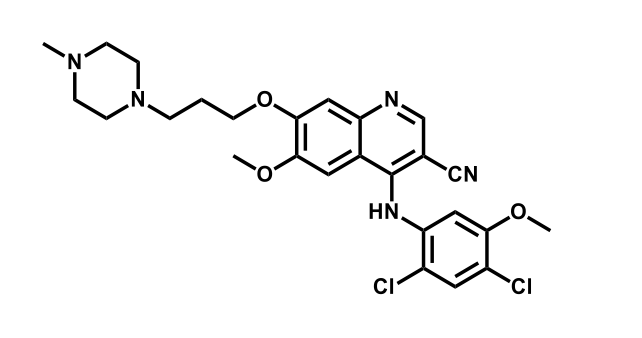
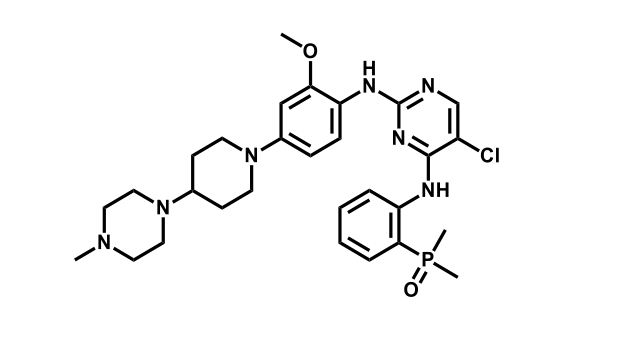
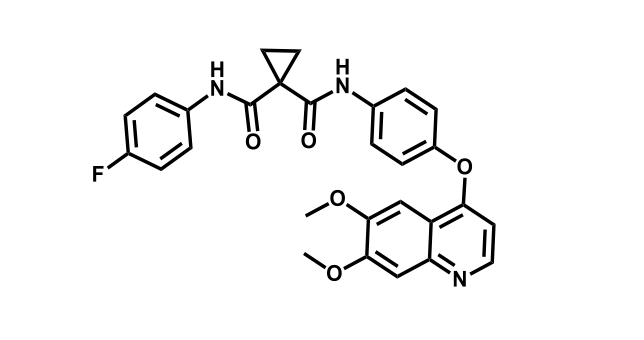
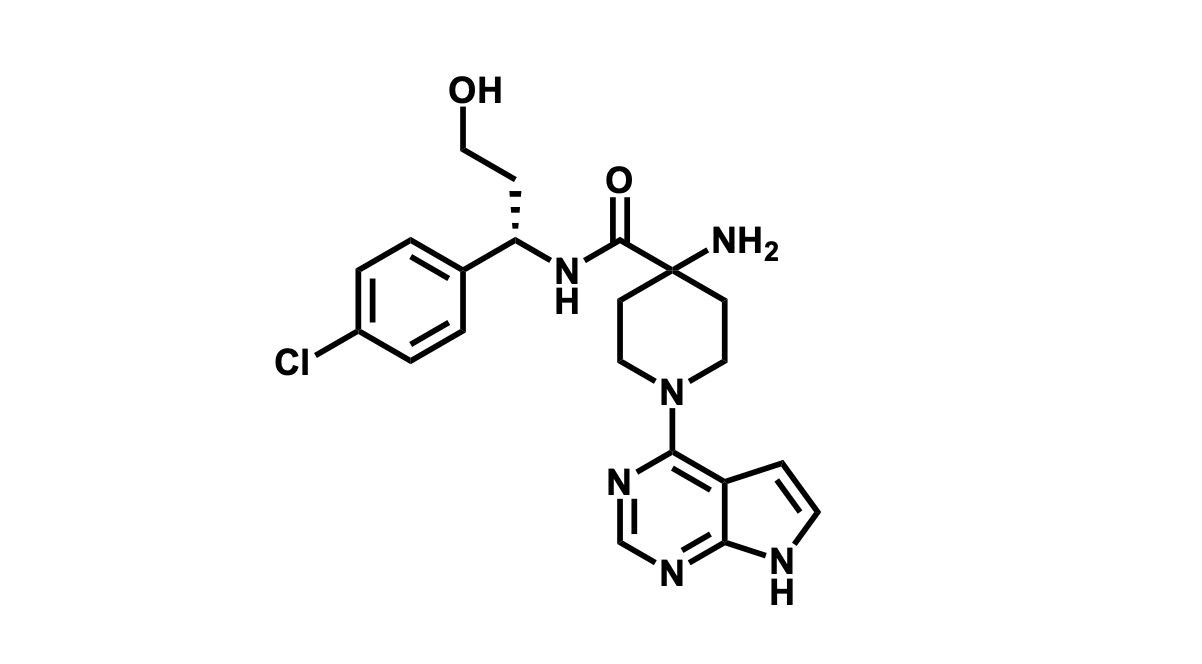
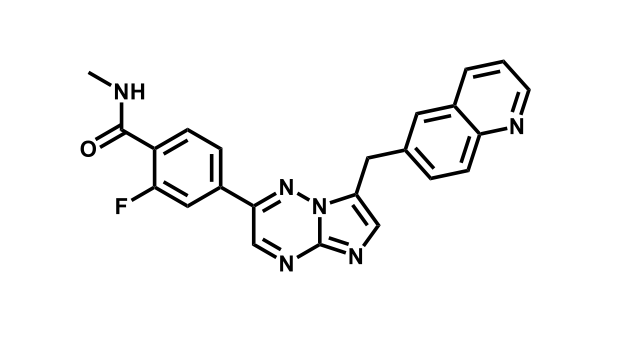
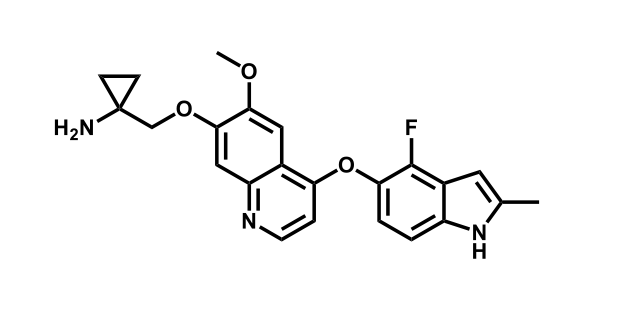
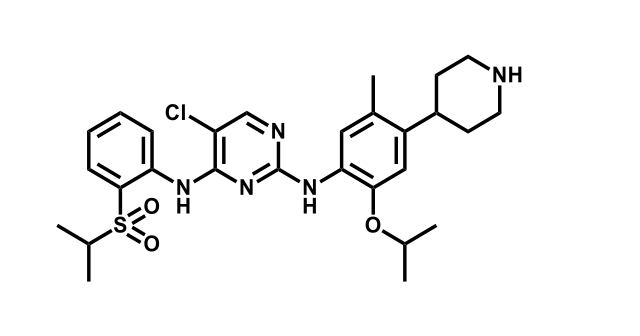
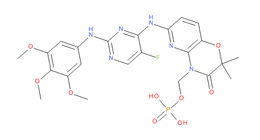
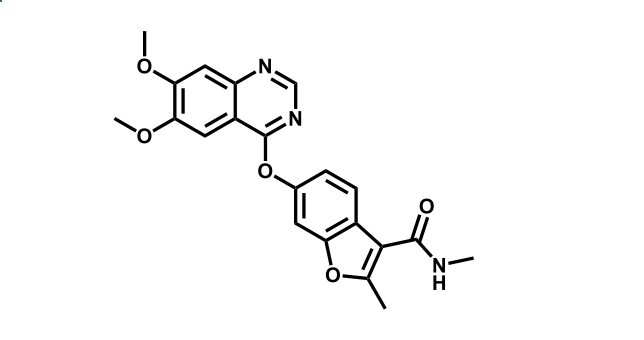
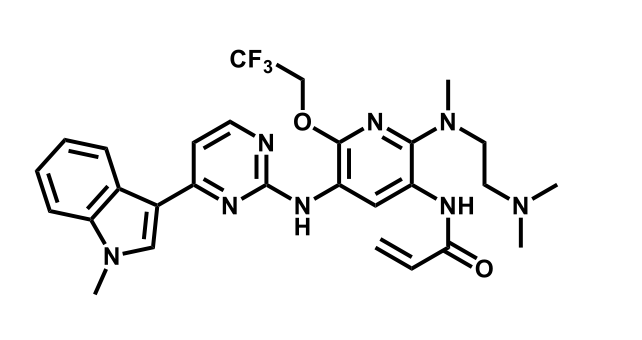
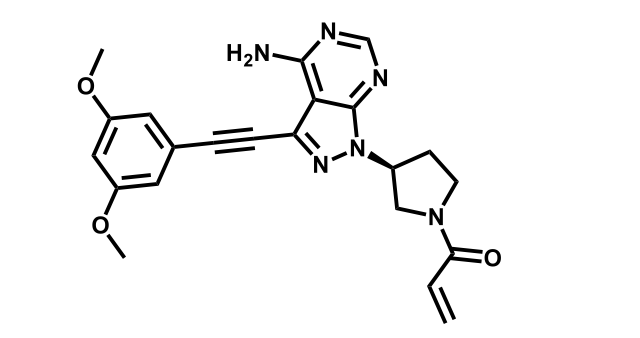
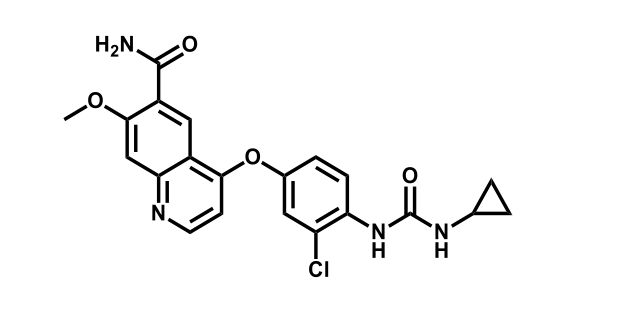
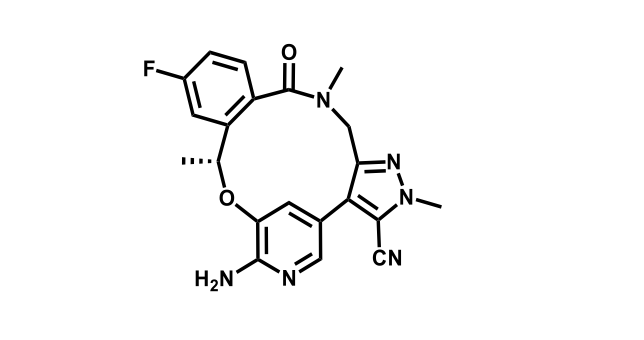
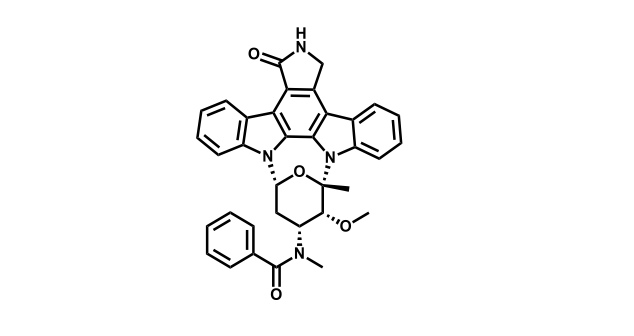
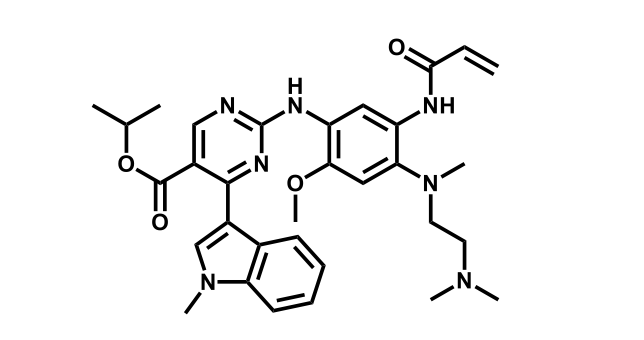
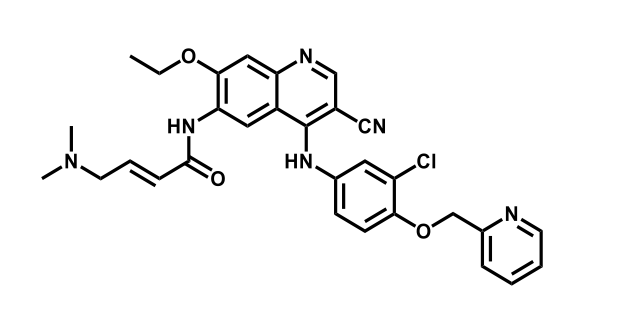
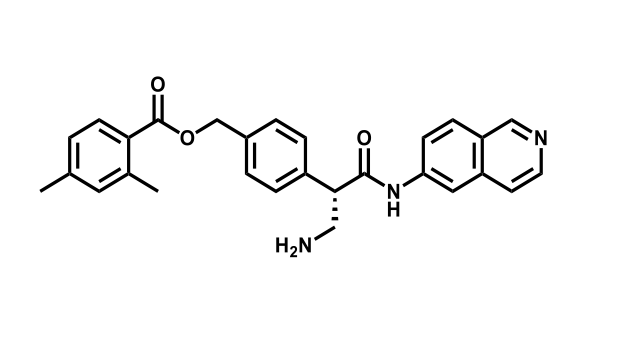
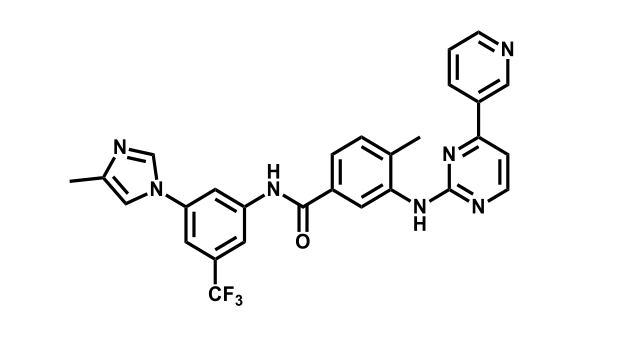
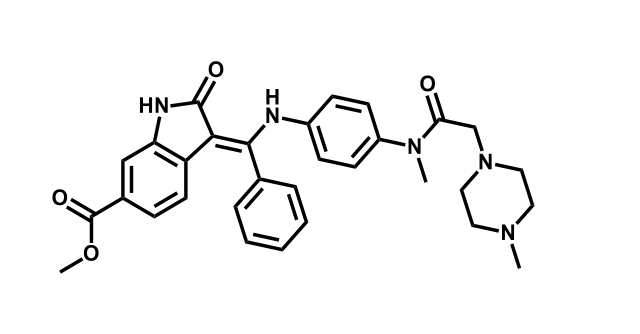
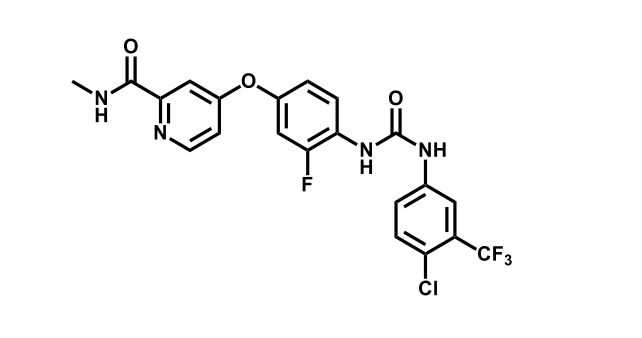
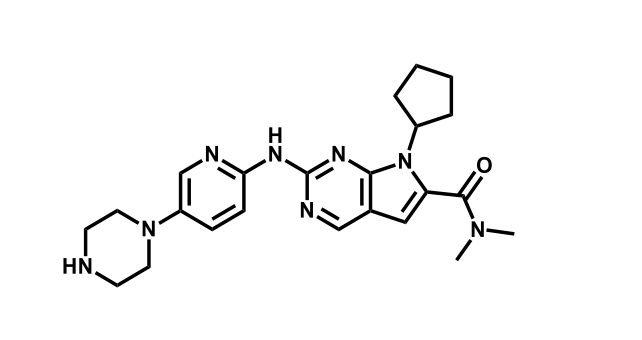
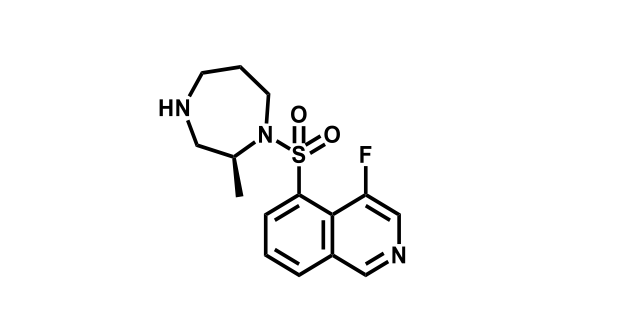
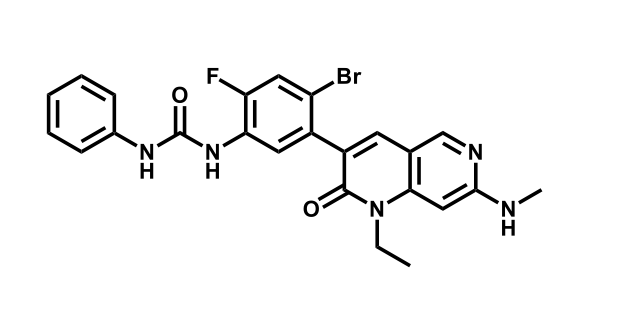
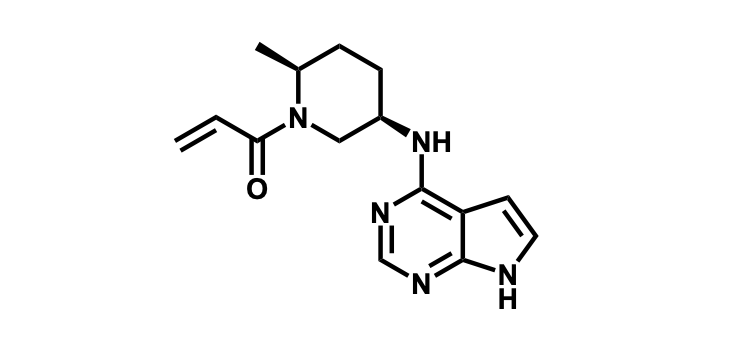
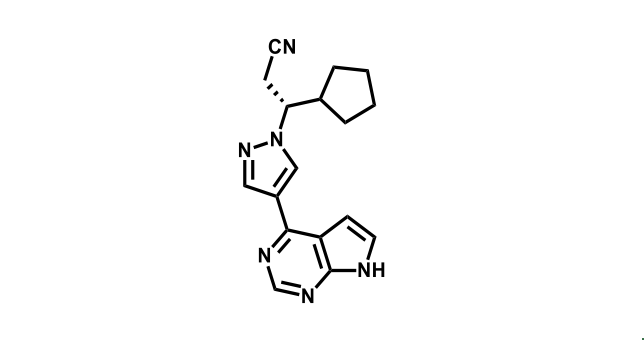
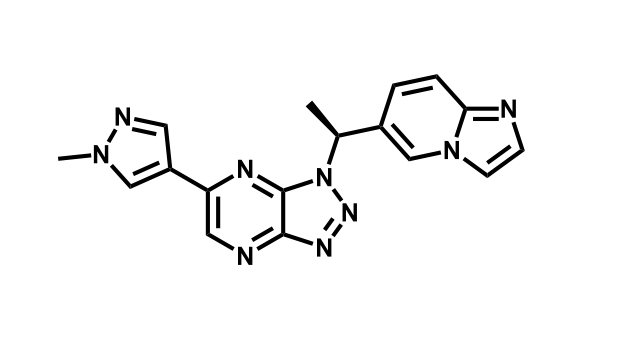
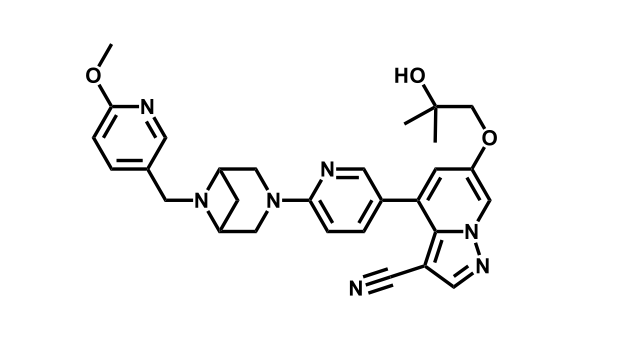
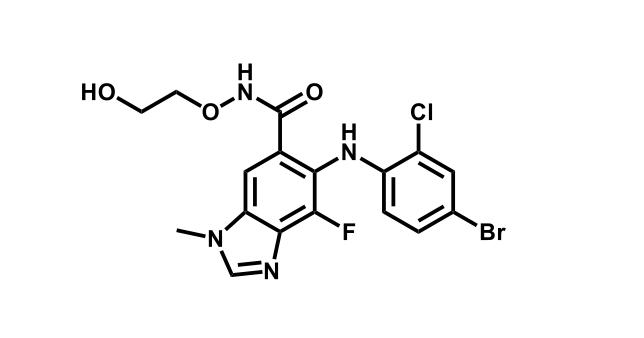
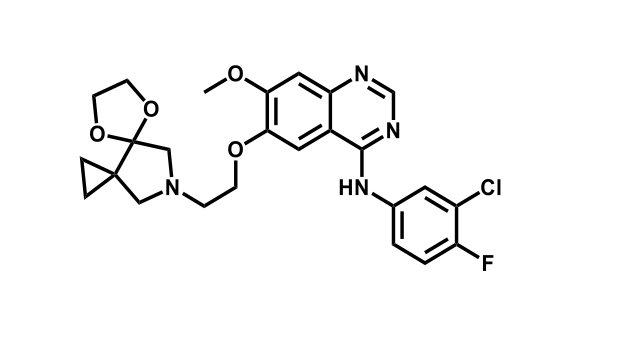
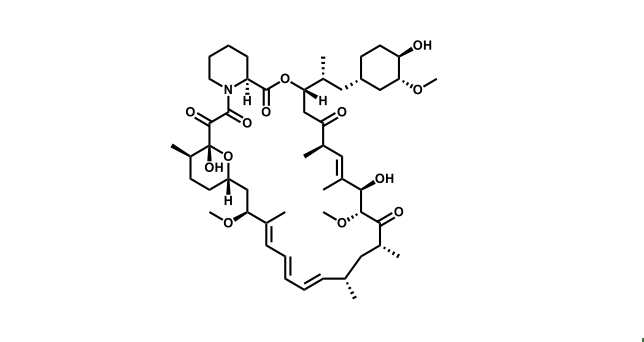
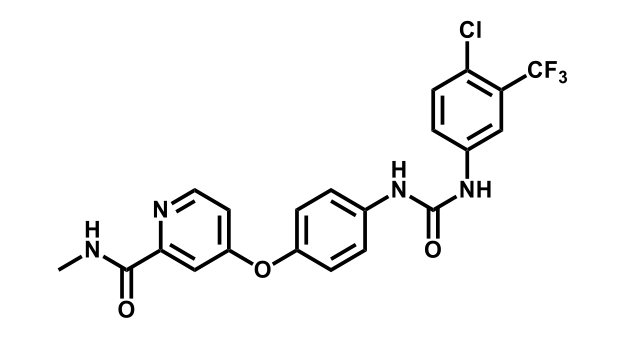
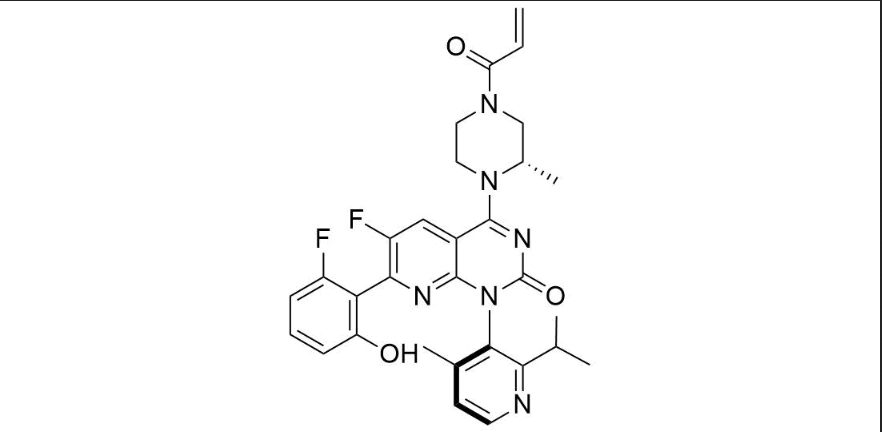
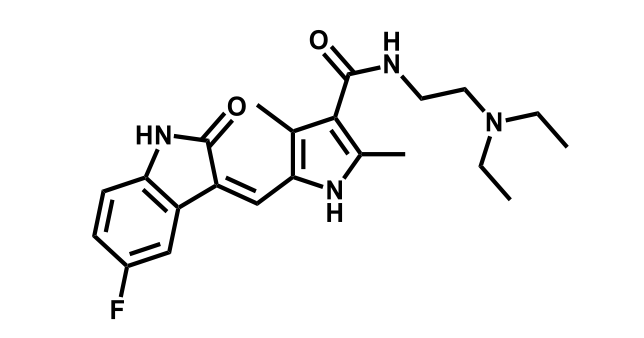
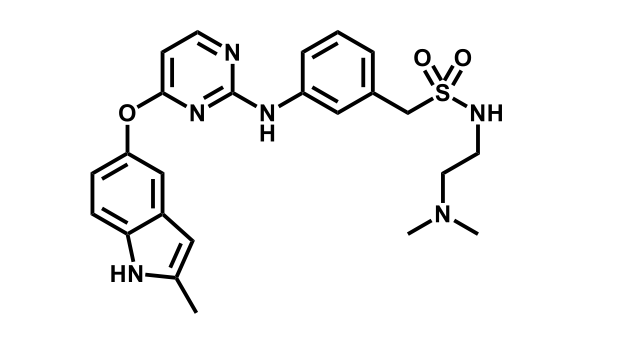
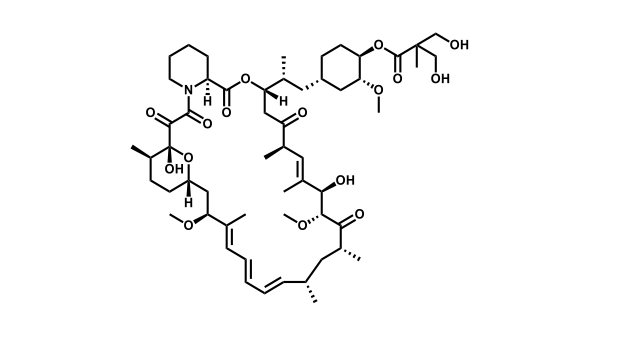
It has been estimated that approximately 30% of current R&D spend in pharmaceutical companies is focused on the development of kinase inhibitors. Protein kinases have become one of the pharmaceutical industry’s most important class of drug target.
To date, 121 drugs targeting protein kinases have been clinically approved (see table below or as a PDF to view structures at a higher resolution). They include Gleevec, an inhibitor of the Bcr-Abl tyrosine kinase, which has transformed chronic myelogenous leukaemia from a disease that was rapidly fatal into a manageable condition.
Advanced (metastatic) breast cancer
Mantle cell lymphoma, CLL
NSCLC with EGFR mutations
NSCLC with ALK translocations
2014 Japan 2015 FDA 2017 EMA
Advanced mutation +ve NSCLC
Breast cancer, HR+ve, HER2-ve
Chronic myelogenous leukemia
PDGFR exon 18 mutation (incl D842V)+ve
Chronic Myelogenous Leukemia
ALK-rearranged metastatic NSCLC
HR+ and PI3K mutation breast cancer
NSCLC with ALK translocations
NSCLC with EGFR mutations
Chronic Myelogenous Leukemia, ALL
ROS1+ve NSCLC, solid tumors NTRK+ve
Advanced metastatic urothelial carcinoma
Chronic myelogenous leukemia
Autoimmune thrombocyto-penia
Chronic immune thrombocytopenia
Metastatic colorectal cancer
Acute myeloid leukemia FLT3 mutant
2018 FDA 2019 EMA 2021 China
chronic lymphocytic leukemia
Chronic Myelogenous Leukemia
Acute myeloid leukemia (AML) and cholangiocarcinoma
Solid tumours with NTRK fusions
Thyroid cancer (DTC) kidney cancer
MHER2-positive breast cancer
Acute myeloid leukemia (FLT3 mutation-positive)
NSCLC with EGFR mutations
HER2-positive breast cancer
Chronic Myelogenous Leukemia
cell carcinomas of the head and neck
Idiopathic pulmonary fibrosis
mantle cell lymphoma CLL, SLL
Advanced (metastatic) breast cancer
2009 FDA, 2010 EMA, MHRA, TGA
Cholangiocarcinoma with FGFR2 fusion
HER2-positive breast cancer
Tenosynovial giant cell tumour
Chronic Myelogenous Leukemia, ALL
Met RET fusion +ve NSCLC MTC
Chronic Myelogenous Leukemia
Clorectal Cancer GIST, HCC
Advanced (metastatic) breast cancer HR+, HER2-ve
Glaucoma ocular hypertension
Advanced GIST Mastocytosis
NSCLC, MTC, thyroid cancers
KRAS non-small-cell lung cancer
Renal Cancer, Imatinib resistant GIST
Advanced Renal Cell Carcinoma
Chronic myelogenous leukemia
M-Melanoma with BRAFV600E
SCLC chemo myelopreservation
HER2-positive breast cancer
Metastatic Melanoma BRAFV600E
The current global market for kinase therapies is about US$20 billion per annum, forecast to increase markedly. There are over 100 small-molecule kinase inhibitors active in late stages of clinical development and many more are likely to be approved in the coming years.
As only about 10% of kinases have been studied in detail there is still much to understand on the roles that protein kinases play in human health and disease.
We believe that development of kinase inhibitors will remain at the forefront of medicine for the foreseeable future.