Researchers from Adrien Rousseau’s lab in the MRC Protein Phosphorylation and Ubiquitylation Unit at the University of Dundee, Scotland, have identified mechanisms contributing to the stress-induced reduction in protein translation using yeast. This new study is now published in EMBO Journal.
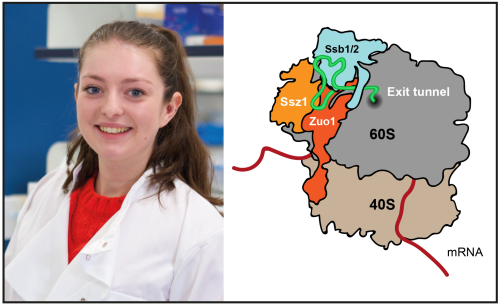
When cells are stressed, they initiate stress responses in order to survive. These responses vary with the stress, but typically involve widespread rewiring of the proteome. General translation is reduced, while proteins used by the cell to fight against the stress are selectively produced. Cells decrease general protein translation, in part, by inhibiting the master regulator of cell growth, TORC1 (Target of Rapamycin Complex 1). In a new study, Ailsa Black from the Rousseau Lab has discovered that the ribosome-associated complex (RAC)/Ssb chaperone system is required to maintain proteostasis and cell viability under TORC1 inhibition.
Ailsa found that in the absence of the Hsp40 cochaperone Zuo1, translation does not decrease in response to loss of TORC1 activity. The functional interaction between Zuo1 and its Hsp70 partner, Ssb, is required for proper translational control and protein homeostasis maintenance upon TORC1 inhibition. Further, she showed that zuo1Δ cells fail to degrade the translation initiation factor eIF4G following TORC1 inhibition, contributing to uncontrolled protein production. Ailsa found that autophagy is defective in zuo1Δ cells, which prevents eIF4G degradation upon TORC1 inhibition. These findings represent a major advancement for our understanding of how translation is modulated in response to stress.
The paper was published on 20th November in EMBO Journal and can be found here (https://doi.org/10.15252/embj.2022113240)

