Researchers at the University of Dundee have investigated the contributions of a key immune communicator molecule, IL-22, in driving colorectal cancer. They made the surprising finding that IL-22 contributes to the initiation of cancer, but once cells are transformed, IL-22 does not affect them. These findings have important implications for immunotherapy of bowel cancer, and suggest that IL-22 is not as good a target as previously suggested.
The cytokine Interleukin-22 (IL-22) plays a critical role in maintaining homeostasis of the intestine with the intestinal microbiota, through its ability to elicit antimicrobial immunity. IL-22 also contributes to mucosal healing and tissue integrity. Multiple recent studies have implicated inflammatory IL-22 in promoting colorectal carcinogenesis; however, it is not clear whether homeostatic levels of IL-22 contribute to intestinal carcinogenesis.
This study by Dr. Yu Chen et al explores the effects of IL-22 on normal and Adenomatous polyposis coli (APC)-deficient small intestinal epithelial cells. The Apc gene is mutated in over 85% of colorectal cancers, and the effects of loss of this gene have been extensively studied in the ApcMin/+ (Adenomatous polyposis coli, multiple intestinal neoplasia) mouse model, in which mutant Apc is expressed from one allele. Loss of heterozygosity at the Apc allele leads to formation of multiple intestinal polyps. This study presents the surprising finding that when APC is mutated, intestinal epithelial cells no longer respond to IL-22.
These data imply that in potentially 85% of colorectal cancers, which express mutant APC, IL-22 is not driving tumour growth. However, the study goes on to show that IL-22 contributes to initial stages of tumour formation, by driving inflammation and DNA damage.
This work was a collaborative study between the labs of Prof. Inke Näthke and Dr. Mahima Swamy, and would not have been possible without combining the different expertise in the two labs. Prof. Näthke’s lab has a long-standing interest in APC function, and how it regulates intestinal epithelial transformation. Dr. Swamy’s lab is interested in how specialised intestinal immune cells “talk” to the intestinal epithelium.
The research was funded by the National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs) and the Wellcome Trust and the Royal Society. It was published in PLOS Biology last week.
https://doi.org/10.1371/journal.pbio.3000540
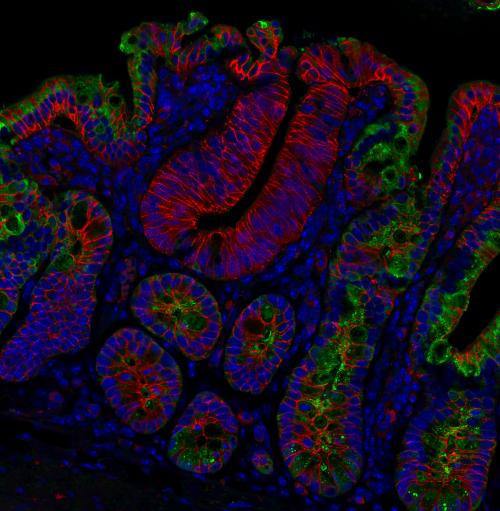
Image legend: This immunofluorescent microscopy image is of small intestinal tissue from an ApcMin/+ (Adenomatous polyposis coli, multiple intestinal neoplasia) mouse that expressed mutant APC. ApcMin/Min polyps, initiated by loss of heterozygosity at the Apc allele and identified by diffuse and increased β-catenin (red), show significantly lower expression of the antimicrobial peptide RegIIIγ (green), an IL-22 target gene, compared to neighbouring ApcMin/+tissue. Cell nuclei are visualized by DAPI staining (blue).

