Spatial and single cell proteomics, signalling pathways and biomarker development in Motor Neuron Disease.
Background: Motor neuron disease (MND) is a rapidly progressive disease affecting upper and lower motor neurons with a lifetime risk of 1 in 300 and a median survival rate of 2-3 years. While genome wide association studies for most common neurodegenerative diseases (ND) have pinpointed the risk alleles that cause familial Parkinson’s, Alzheimer’s, and motor neurone disease. However, several of these diseases appear to arise due to sporadic mutations as well, thus a better understanding and dissecting the signalling pathways would be the major focus in developing personalised therapeutic strategies in treating these devastating diseases. Most ND do share commonalities in their signalling and associated disease co-pathologies, for example, loss of function and aggregation seen in >97% ALS, 30 to 50% in FTD and AD patients suggesting a commonality at the level of molecular and signalling contributing to the disease progression. Recent research undertaken to study the role of glia, a vital immune and supporting cells of brain and specific population of neuronal cells such as Dopaminergic, cholinergic neurons within brain by global and single cell RNASeq. However, a thorough understanding of proteins that play a key function of a cell is inadequate, an un-biased mapping of proteomic and posttranslational modification (PTMs) profiles would aid in better understanding of the disease progression.
My research focus is to study and understand signalling pathways of motor neuron disease (MND) (aka, Amyotrophic lateral Sclerosis (ALS) with a precise interest to study TDP-43 and C9orf72 proteins. We are keen on developing a framework of quantitative proteomic methodologies to study the role of TDP-43 and C9orf72 loss of function (LoF) and gain of toxicity in MND. To tackle these research questions, our lab employs human induced-pluripotent stem cells (hiPSC) models, CRISPR-CAS9-based genome editing, biochemistry and state-of-the-art Ultra-sensitive mass spectrometry including single cell proteomic workflows and Bioinformatics. Additionally, we employ miniaturized proteomic methodologies to enrich post translational modifications such as protein phosphorylation and Ubiquitylation from sub-microgram (5 to 10µg) starting materials derived from cell/tissues including sub-cellular organelles.
Current research focus on 3 major areas includes but not limited to:
1. Development of single cell and spatial proteomics methodologies to study motor neuron disease and other neurodegenerative diseases: The complexity and cellular heterogeneity of brain is being studied to understand the brain function, neurodegeneration and regeneration using RNASeq at single cell level. We aim to utilize and develop Ultra-sensitive mass spectrometry methodologies to define the proteomes at single cell resolution to study MND and other neurodegenerative disease.
2. Investigate and study signalling pathways of TDP-43 and C9orf72 loss-of-function and gain of toxicity in motor neuron disease. We will employ Organelle proteomics (lysosome and mitochondria) and PTM (Phosphorylation and Ubiquitinome) enrichment methodologies on hiPSC motor neurons and glial cell models in studying cell signalling of TDP-43 and C9orf72 proteins in MND.
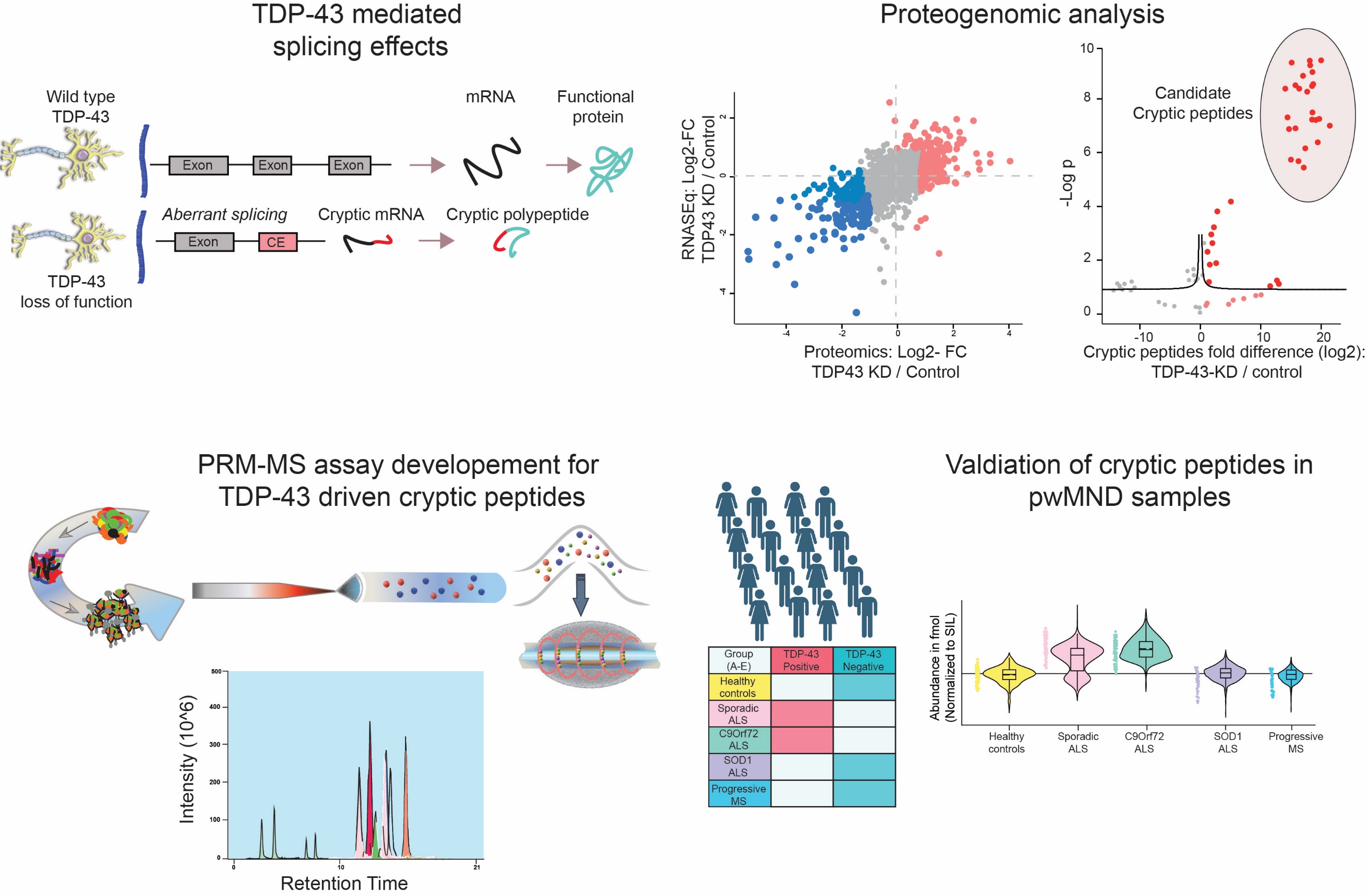
3. Pinpointing changes in splicing mediated by TDP-43 Loss-of-function in clinical samples with a goal to develop blood-based biomarkers for motor neuron disease: TDP-43 functions to regulate splicing and RNA stability. Loss of function of TDP-43 often observed in familial as well as sporadic ALS results in aberrant splicing on plethora of genes leading to the generation of small non-functional cryptic exons/peptides. We are developing MS-based targeted parallel reaction monitoring assays to identify and quantify absolute amounts of cryptic peptides in blood, CSF and in post-mortem derived brain samples. The developed assays will serve as diagnostic/prognostic biomarkers as well as clinical management of MND.
Collaborators:
Prof. Siddharthan Chandran, Prof. Suvankar Pal and Dr. Bhuvaneish Selvaraj (UK-DRI at Edinburgh, University of Edinburgh and Anne Rowling regenerative Neurology Clinic)
Prof. Miratul Muqit (MRC PPU)