Key Facts
Bio:
Ruben studied Physics at the Complutense University of Madrid (Spain). Afterwards, he carried out a research fellowship in the lab of Marcellus Ubbink at Leiden University (The Netherlands), which sparked his curiosity for structural biology. For his PhD, he joined the department of Wolfgang Baumeister at the Max Planck Institute of Biochemistry (Germany), where he investigated the structure of the presynaptic terminal by cryo-electron tomography (cryo-ET) [see e.g. Fernandez-Busnadiego et al., J Cell Biol 2010, 2013]. To dive deeper into the molecular mechanisms of neuronal function, he worked with Pietro De Camilli (Yale University, USA) as a postdoctoral fellow [see e.g. Fernandez-Busnadiego et al., PNAS 2015]. Later, he returned to the Max Planck Institute of Biochemistry to start a group using cryo-ET to investigate in situ the structure of toxic protein aggregates within cells. This work provided important insights into the structural mechanisms underlying neuronal death in neurodegenerative diseases [see e.g. Bäuerlein et al., Cell 2017; Guo et al., Cell 2018; Guo et al., Nature 2018; Trinkaus et al., Nat Comm 2021; Riemenschneider et al., EMBO Rep 2022]. In 2019, he joined the faculty of the University Medical Center Göttingen (Germany), where he continues to harness the latest electron microscopy technology to unravel the structural basis of cell function and pathological dysfunction.
Abstract:
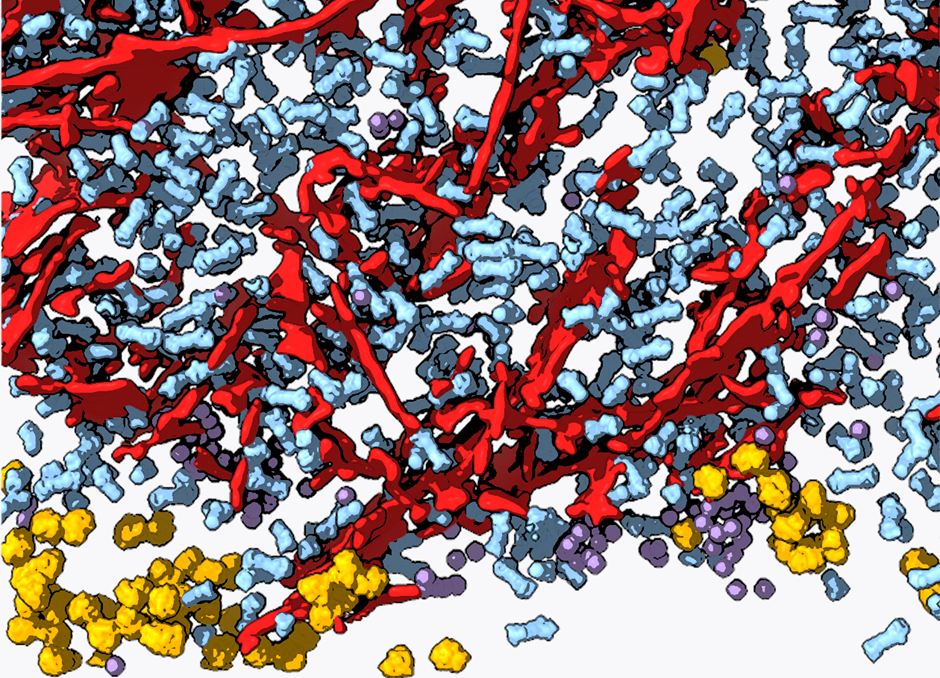
Protein aggregation is a hallmark of many neurodegenerative diseases, including Huntington’s, Parkinson’s and amyotrophic lateral sclerosis. However, the mechanisms linking aggregation to neurotoxicity remain poorly understood, partly because only limited information is available on the native structure of protein aggregates inside cells. We address this pressing issue utilizing the latest developments in cryo-electron tomography (cryo-ET). We use cryo-focused ion beam to prepare thin lamellas of vitrified cells containing protein aggregates, and subsequently image them in three dimensions by cryo-ET. This allows us to analyse aggregate structure within pristinely preserved cellular environments and at molecular resolution [1, 2, 3]. Here, I will discuss how our latest results shed new light into the cellular mechanisms of neurodegeneration.
References [1] Bäuerlein et al. and Fernández-Busnadiego, Cell (2017) 171 (1), 179-187 [2] Guo et al. and Fernández-Busnadiego, Cell (2018) 172 (4), 696-705 [3] Trinkaus et al. and Fernández-Busnadiego, Nat Comm (2021) 12 (1), 2110