Hannah Tovell, a PhD student in Dario Alessi's lab, working in collaboration with Claire Crafter (AstraZeneca) and Alessio Ciulli and Andrea Testa in the Ciulli lab, have elaborated a compound that we have termed SGK3-PROTAC1 that induces selective degradation of SGK3 protein kinase.
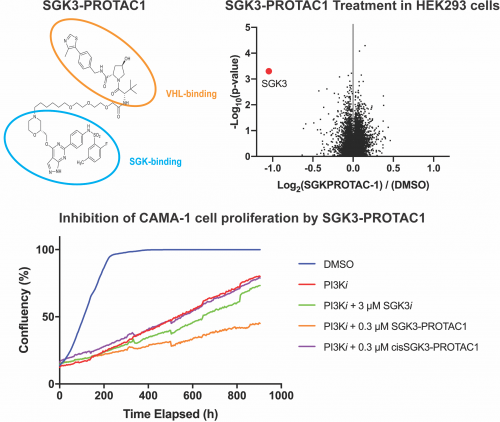
Hannah was able to show that SGK3-PROTAC1 suppressed proliferation of ZR-75-1 and CAMA-1 cancer cell lines treated with a PI3K inhibitor (GDC0941) more effectively than could be achieved by a conventional SGK isoform inhibitor (14H), underscoring the benefit of the PROTAC approach.
0.3 µM SGK3-PROTAC1 induced 50% degradation of endogenous SGK3 within 2 hours, with maximal 80% degradation observed within 8 hours, accompanied by a loss of phosphorylation of NDRG1, an SGK3 substrate. SGK3-PROTAC1 did not degrade closely related SGK1 and SGK2 isoforms that are nevertheless engaged and inhibited by SGK3-PROTAC1.
Proteomic analysis undertaken by Houjiang Zhou revealed that SGK3 was the only cellular protein whose cellular levels were significantly reduced following treatment with SGK3-PROTAC1 (see Figure).
We have also developed a negative control compound termed cisSGK3-PROTAC1 that is an epimer analogue incapable of binding to the VHL E3 ligase that has no impact on SGK3 stability. We recommend that in all future biological studies the effects that SGK3-PROTAC1 have on biology be compared side by side with cisSGK3-PROTAC1.
SGK3 plays a key role in mediating resistance of breast cancer cells to Class 1 PI3K or Akt inhibitors, by substituting for loss of Akt activity and restoring proliferative pathways such as mTORC1 signaling. SGK3-PROTAC1 will be an important reagent to explore the roles of the SGK3 pathway in biology and in mediating resistance to PI3K pathway therapy.
To read Hannah’s paper click here.

