The Alessi lab at the MRC Protein Phosphorylation and Ubiquitylation Unit (MRC PPU) has been studying the regulation and function of the WNK (With No Lysine) signalling pathway for many years, which plays a critical role in regulating ion homeostasis and blood pressure in humans.
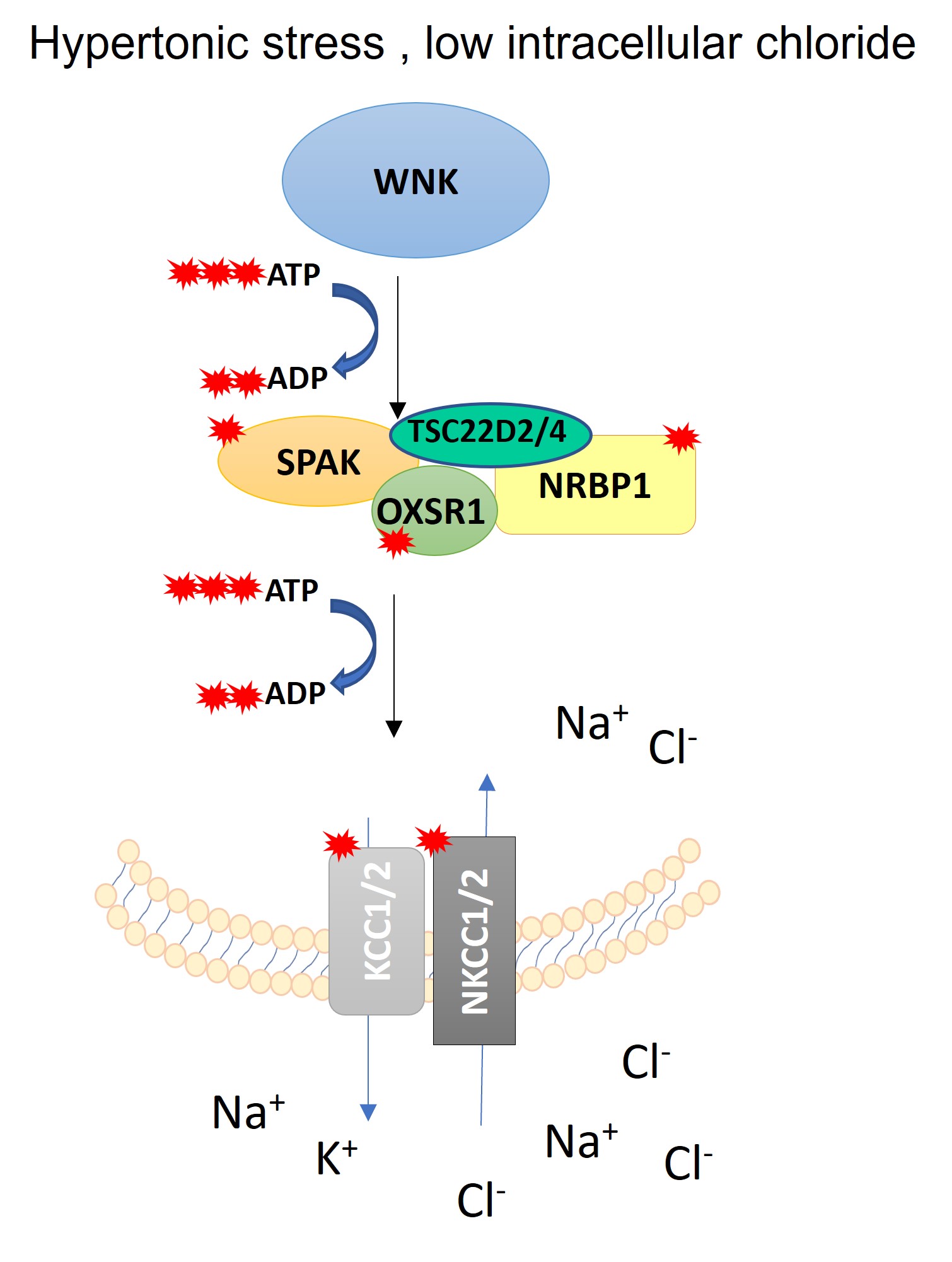
In work initiated by Dr Toby Dite (currently at the WEHI, Melbourne) and led by Dr Ramchandra Amnekar, the team has found that osmotic stress in cells induced the WNK kinases to physically interact with a family of pseudokinases known as NRBP1 and NRBP2.
Although these proteins share structural similarities with kinases, they lack essential catalytic motifs required for phosphorylation. Intriguingly, the study reveals that NRBP1 directly binds to the kinase domains of WNK1 , but only when cells are treated with osmotic stress.
Using CRISPR knockout and inducible targeted degradation of NRBP1 strategies, the researchers show that loss of NRBP1 profoundly impairs WNK pathway activation in mammalian cells in basal conditions and in response to osmotic stress particularly impacting WNK1 kinase activity and its phosphorylation of downstream components. These findings position NRBP1 and NRBP2 as critical upstream activators of the WNK pathway.
Remarkably, NRBP1 and NRBP2 are closely related in sequence to the WNK isoforms, raising the possibility that they could be considered WNK5 and WNK6. Like WNK kinases and their downstream targets SPAK and OXSR1, NRBP1 and NRBP2 contain a C-terminal Conserved (CCT) domain which is structurally similar to WNK pathway components.


Through AlphaFold3 structural modelling and targeted mutagenesis, the team has developed a 3D model in which WNK1, SPAK, NRBP1, and two additional NRBP1-interacting proteins TSC22D2 and TSC22D4, form a major complex via their conserved CCT domains. These domains appear to recognise and bind to R-hydrophobic motifs, which are conserved across the complex.
Excitingly, collaborative work led by the group of María Castañeda-Bueno in Mexico City, demonstrated that NRBP1, TSC22D2, and TSC22D4 play a pivotal role in regulating the physiology of the distal convoluted tubule in the kidney by modulating WNK4 pathway activity and its key downstream effector, NCC (the Na⁺-Cl⁻ co-transporter). This provides strong evidence for the physiological relevance of this pathway.
While these findings significantly advance our understanding of WNK signalling, key questions remain, including how osmotic stress is sensed by cells and how it promotes the interaction of NRBP1 and TSC22D4 with WNK1/4 to drive activation of the pathway.
To Read a copy of the discussed papers click here