Ex MRC-PPU student, Darren Cross plays major role in developing new lung cancer drug
Darren Cross who undertook is PhD training within the MRC-PPU working in Philip Cohen's laboratory (1993-1997) has played a major role in developing a new drug termed TAGRISSOââ€_¢ (AZD9291) that was recently approved (13 November 2015) by the US Food and Drug Administration for the treatment of metastatic non-small cell lung cancer.
AZD9291 is a novel EGF Receptor (EGFR) inhibitor that does not significantly affect the wild type but potently inhibits the common cancer T790M mutation that renders the EGFR resistant to many of the other EGFR inhibitor drugs that are on the market.
Darren Cross led the group in AstraZeneca that initially developed AZD9291 and published the first paper on this compound in 2014:
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, Hughes G, Rahi A, Jacobs VN, Red Brewer M, Ichihara E, Sun J, Jin H, Ballard P, Al-Kadhimi K, Rowlinson R, Klinowska T, Richmond GH, Cantarini M, Kim DW, Ranson MR, Pao W. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014 Sep;4(9):1046-61. Click here for copy of paper
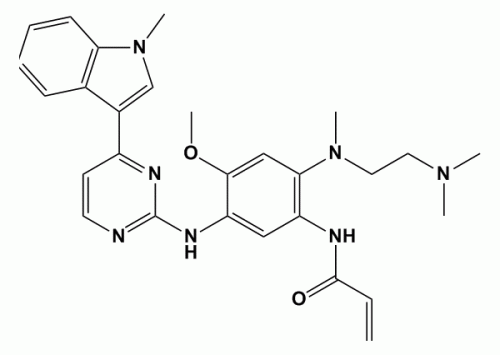
The structure of TAGRISSOââ€_¢ (AZD9291) is shown in the above Figure. It works by forming a covalent bond to a kinase domain Cys797 residue in mutant T790M-EGFR. The property of TAGRISSOââ€_¢ (AZD9291) not significantly inhibiting wild type EGFR is very favourable and makes it possible to achieve a therapeutic dose of compound in patients that inhibits the cancer driving function of the T790M-EGFR mutant, without causing side effects that result from inhibition of wild type EGFR.
TAGRISSOââ€_¢ (AZD9291) displays a response rate of 59% in patients with T790M EGFR metastatic non-small cell lung cancer and duration of response of 12.4 months.
TAGRISSOââ€_¢ (AZD9291) was one of fastest development programmes in the history of pharmaceutical research – from start of clinical trials to approval in just over two and a half years.
For more information click here
AZD9291 is a novel EGF Receptor (EGFR) inhibitor that does not significantly affect the wild type but potently inhibits the common cancer T790M mutation that renders the EGFR resistant to many of the other EGFR inhibitor drugs that are on the market.
Darren Cross led the group in AstraZeneca that initially developed AZD9291 and published the first paper on this compound in 2014:
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, Hughes G, Rahi A, Jacobs VN, Red Brewer M, Ichihara E, Sun J, Jin H, Ballard P, Al-Kadhimi K, Rowlinson R, Klinowska T, Richmond GH, Cantarini M, Kim DW, Ranson MR, Pao W. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014 Sep;4(9):1046-61. Click here for copy of paper
The structure of TAGRISSOââ€_¢ (AZD9291) is shown in the above Figure. It works by forming a covalent bond to a kinase domain Cys797 residue in mutant T790M-EGFR. The property of TAGRISSOââ€_¢ (AZD9291) not significantly inhibiting wild type EGFR is very favourable and makes it possible to achieve a therapeutic dose of compound in patients that inhibits the cancer driving function of the T790M-EGFR mutant, without causing side effects that result from inhibition of wild type EGFR.
TAGRISSOââ€_¢ (AZD9291) displays a response rate of 59% in patients with T790M EGFR metastatic non-small cell lung cancer and duration of response of 12.4 months.
TAGRISSOââ€_¢ (AZD9291) was one of fastest development programmes in the history of pharmaceutical research – from start of clinical trials to approval in just over two and a half years.
For more information click here

