The organization of our genomes into chromatin controls the degree of DNA accessibility, which in turn regulates which genes are expressed. This is essential to preserves the identity and stability of our cells. It is therefore not surprising that mutations in the proteins that make up chromatin are linked to human diseases such as cancer and neurodevelopmental disorders. Histone chaperones play a central role in regulating chromatin structure during DNA replication, transcription, and DNA repair, and they do so by binding histones and escorting them from their biogenesis in the cytoplasm to their incorporation into chromatin. Despite decades of work since the first histone chaperones were identified, new functions of these proteins are still emerging.
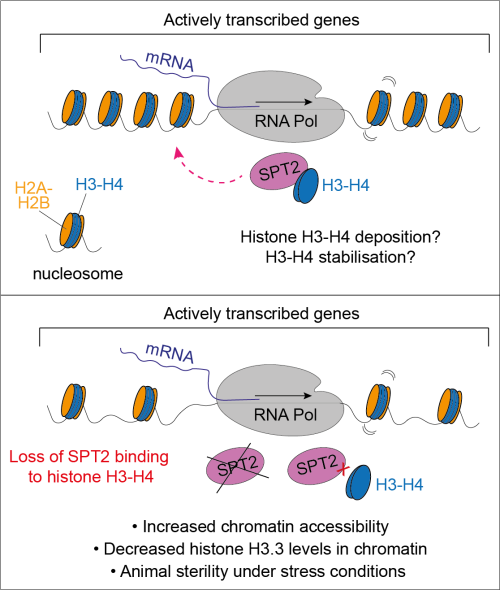
In a recent publication in Nature Structural & Molecular Biology, John Rouse’s lab used a combination of genetics, genomics, and biochemistry – in human cells and the Caenorhabditis elegans model system – to understand how the histone chaperone SPT2 affects chromatin structure and organism fitness. Giulia Saredi, the postdoc who led the study, showed that SPT2 is required to restrict chromatin accessibility at highly transcribed genes and to protect the germline tissue of nematodes grown under stress conditions. Mechanistically, this function of SPT2 relies on its ability to bind histones H3-H4, as a single amino acid substitution in SPT2 that reduces binding to H3-H4 phenocopies the loss of the entire gene. In line with this, human cells expressing a histone-binding deficient version of SPT2 show defective incorporation of the histone variant H3.3 into chromatin. This work relied on excellent national and international collaborators, which proved to be crucial to address different aspects of the project – underscoring the importance of open communication and collaboration in science.
With several aspects of SPT2 biology still puzzling, this work sets the stage to further shed light onto how chromatin assembly and maintenance contribute to preserving genome stability.
This work was supported by grants from the Medical Research Council (UK), Wellcome Trust, Cancer Research UK, the European Research Council, and the Korean Institute for Basic Science. Giulia Saredi was supported by an EMBO Long Term Fellowship, a Marie Skłodowska-Curie Individual Fellowship and a SULSA Early Career Researcher Fund.

