Derailment of the PI3K signalling network contributes to many human diseases including cancer.
Recent work undertaken mainly by Ruzica Bago in the Alessi lab has revealed that a poorly studied kinase related to Akt termed SGK3, is recruited and activated at endosomes, by virtue of its PX domain binding to 3-phosphoinositide (PtdIns(3)P-see http://www.biochemj.org/content/463/3/413.long). Our initial data suggested that two types of phosphoinositide 3-kinase (PI3K) termed Class I and Class III were responsible for generating the PtdIns(3)P that triggered SGK3 activation.
Other work undertaken by Ruzica Bago revealed that SGK3 activity was upregulated in response to cancer therapies that target the Class I PI3K and Akt pathways (http://emboj.embopress.org/content/35/17/1902.long).
Nazma Malik a PhD student in the Alessi lab, set out to study how endogenous SGK3 was regulated in cells. She discovered that SGK3 was rapidly activated over 10-fold by growth factors such as IGF1.
Employing selective inhibitors, Nazma was able to demonstrate that both Class 1 and Class 3 PI3Ks were involved in triggering activation of SGK3 by growth factors. She found that in order to inhibit SGK3 activation in response to growth factors it was necessary to block both Class I and Class III PI3Ks.
Nazma then undertook a series of localisation studies with help of Alan Prescott, that revealed that IGF1 significantly enhances endosomal PtdIns(3)P levels in a manner that was blocked using specific inhibitors of the Class III PI 3K.
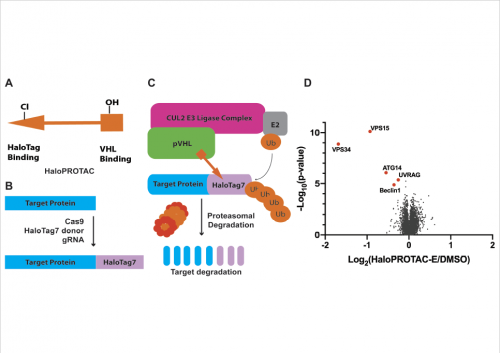
To obtain further evidence for the growth factors stimulating PtdIns(3)P via activation of Class III PI3K, Nazma employed a new technology developed within the Sapkota lab at the MRC-PPU for targeting endogenous proteins for degradation, called affinity-directed protein missile system (see http://rsob.royalsocietypublishing.org/content/7/5/170066).
Nazma used this approach to knock-down the endogenous catalytic subunit of Class III PI3K (hVPS34) and one of its critical regulatory subunits (UV-Rag). The results from these experiments confirmed the important role that Class III PI3K plays in regulating SGK3 in conjunction with the Class I PI3 kinase.
Further work is need to work out the molecular mechanism by which the Class III PI3K is activated by IGF1.
Karen Anderson at the Babraham Institute in Cambridge, undertook critical phosphoinositide measurement experiments that also supported the notion that IGF1 promotes activation of SGK3 via the Class 1 PI3K stimulating production of PtdIns(3)P (from the sequential dephosphorylation of PtdIns(3,4,5)P3 to PtdIns(3,4)P2 and then to PtdIns(3)P).
However, all of the IGF1 induced PtdIns(3)P appears to be localised on the endosome and not the plasma membrane where PtdIns(3,4,5)P3 to PtdIns(3,4)P2 reside. Therefore, it is not clear where the PtdIns(3)P produced from activation of class 1 PI3K resides and whether this translocated to the Golgi or is simply too dilute at the plasma membrane to be visualised.
Nazma’s work suggests that Ptd(3,4,5)P3 produced from the activation of Class 1 PI3K could contribute to SGK3 activation by promoting mTORC2 to phosphorylate SGK3.
Finally, Nazma also found that oncogenic Ras also activated SGK3, but this occurred solely through the Class 1 PI3K pathway.
Nazma’s findings highlight the versatility of upstream pathways that activate SGK3. The also help explain why SGK3 activity can remain active and counteract physiological conditions or stresses where either Class 1 or Class 3 PI3K pathways are inhibited.
To read a copy of Nazma’s paper click here.

