PI3K and Akt inhibitors are being evaluated in clinical trials for the treatment of human tumours including breast cancer that display driver mutations that inappropriately elevate the PI3K/Akt signalling pathway. Despite initial promise, the majority of these tumours rapidly evolve to resist the PI3K/Akt pathway therapy.
Ruzica Bago, a postdoc based in the Alessi working in close collaboration with researchers at Memorial Sloan Kettering Cancer Centre, AstraZeneca, and the University of Liverpool, set out to decipher intrinsic signalling mechanisms that account for adaptive resistance to PI3K/Akt pathway suppression.
Building upon work carried out by a previous MRC-PPU PhD student Eeva Sommer, Ruzica showed that prolonged treatment of a panel of breast cancer cells lines (ZR-75-1, CAMA-1,T47D and BT-474 ) for over 2-days with with PI3K or Akt inhibitors, led to a marked increase in the expression and activity of a poorly studied protein kinase termed the serum and glucocorticoid-regulated kinase-3 (SGK3), that is closely related to Akt and also activated by the same upstream kinases (PDK1 and mTORC2).
Akt kinase possesses a PH domain at its N-terminus that binds to the lipid second messenger PtdIns(3,4, 5)P3 product generated by PI3K, which induces a conformational change promoting phosphorylation and activation of Akt by PDK1 and mTORC2.
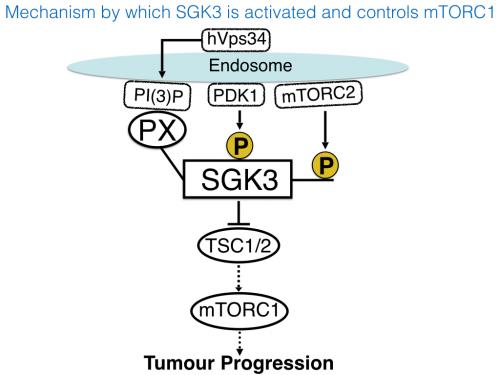
In contrast, SGK3 instead of a PH domain at its N-terminus possesses another lipid interacting motif termed a PX domain, that binds to PtdIns(3)P-and not PtdIns(3,4, 5)P3. SGK3 is the only known protein kinase to possess a PtdIns(3)P binding domain.
Ruzica discovered that SGK3 was activated in an analogous mechanism to Akt namely that PtdIns(3)P-binding to the PX domain promoted PDK1 phosphorylation and hence activation of SGK3. This effect is prevented by introducing a mutation within the PX domain that prevents SGK3 from binding to PtdIns(3)P.
These findings raised the question as to what was the identity of the lipid kinase in the cell that generates the PtdIns(3)P that stimulates the activation of SGK3 in breast cancer cell lines treated with PI3K or Akt inhibitors. Employing structurally diverse highly selective inhibitors, Ruzica experiments strongly point towards an enzyme termed hVps34, which is one of the major lipid kinase in the cell that generates PtdIns(3)P that is located at endosomes of cells at the same location SGK3 residues.
To better dissect SGK3 signalling, Ruzica also characterized a recently reported Sanofi SGK1 inhibitor termed 14h that she observed potently inhibited SGK3 with an IC50 of ~ 3nM. Although 14h was reported to act as an ATP completive inhibitor, Ruzica noticed that in addition to suppressing SGK3 activity, 14h also prevents the phosphorylation of SGK3 by PDK1 and mTORC2 pathway and hence its activation of SGK3 in cells. Consistent with 14h inhibiting the activation of SGK3 in cells, Ruzica demonstrated that in vitro 14h prevents PDK1 from phosphorylating and activating SGK3 in the presence of PtdIns(3)P in vitro.
Given the similarity between Akt and SGK3, Ruzica speculated that these kinases could phosphorylate an overlapping set of substrates. Her data suggested that this seems to be the case, as ether prolonged inhibition of PI3K/Akt enabled SGK3 to fully re-activate the mTORC1 signalling pathway by phosphorylating TSC2. Under these conditions mTORC1 activation is now blocked by 14h SGK inhibitor.
Employing an Akt phosphorylation motif antibody-Ruzica found that out of 9 Akt substrates identified in a cell extract 6 of these were likely to be phosphorylated by both Akt and SGK3
Lastly, Pau Castel working in the laboratory of José Baselga working at the Memorial Sloan Kettering Cancer Center demonstrated that a combination of Akt (MK-2206) and SGK (14h) inhibitors induced marked regression of a breast cancer (BT-474) cell derived tumours in a nude mouse xenograft model, under conditions where either inhibitor administered individually had minimal effects.
These results highlight the importance of the hVps34-SGK3 pathway and suggest it represents a major mechanism, which cells utilise to counteract inhibition of PI3K-Akt signalling. They also provide novel mechanistic insights of how PtdIns(3)P can stimulate activation of SGK3.
The characterisation of the 14h SGK inhibitor suggests that it will become a useful research tool to probe biology controlled by SGK isoforms. 14h represents a valuable addition to our growing arsenal of signal transduction inhibitors to dissect functional roles of protein kinases.
Finally, and perhaps most importantly findings described in Ruzica’s paper highlight the therapeutic potential of a strategy targeting both the Akt and SGK kinases for the treatment of cancer.
To read a copy of Ruzica’s paper published in the EMBO J. click here.

