Sourav Banerjee's research uncovers remarkable interplay between NUAK1 and PLK1 kinases
Much research in the MRC-PPU has focused on understanding the regulation and function of two related protein kinases termed NUAK1 and NUAK2 that are activated by the LKB1 tumor suppressor. LKB1 phosphorylates these enzymes at their T-loop residues stimulating their activity. Little is known about the NUAK enzymes, although some initial work indicates that they are likely to play diverse roles including controlling cell survival, senescence, adhesion and polarity.
Only a single substrate, namely the MYPT1 subunit of the PP1Ã_Â_MYPT1 myosin phosphatase complex, has been reported. This finding was made by Anna Zagorska who previously undertook her PhD in the Alessi lab in the MRC-PPU. Anna's data suggested that NUAK1 phosphorylation of PP1Ã_Â_MYPT1 triggered binding to 14-3-3 isoforms and lead to an inhibition of phosphatase activity.
Previous work by other groups has also revealed that PP1Ã_Â_MYPT1 acts to inactivate PLK1 by dephosphorylating the T-loop Thr210 residue, thereby controlling mitosis. The ability of PLK1 to interact with PP1Ã_Â_MYPT1 is dependent upon phosphorylation of MYPT1 at Ser473 by the cyclin dependent protein kinase-2 (CDK2), which creates a docking site recognised by the Polo-box domain of PLK1. Interestingly, Ser473 lies immediately adjacent to the NUAK1 phosphorylation site (Ser472) that controls 14-3-3 binding. This therefore suggests that phosphorylation of MYPT1 by NUAK1 and 14-3-3 binding could directly interfere with the ability of PP1Ã_Â_MYPT1 to interact with and hence dephosphorylate PLK1.
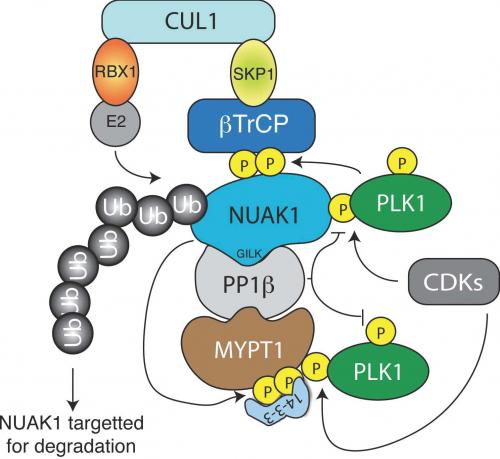
Intrigued by all of this, a PhD Student in Alessi lab, Sourav Banerjee, has spent the last few years exploring links between NUAK1 and components that regulate cell cycle and PLK1. This research has lead Sourav to discover that expression of NUAK1 is controlled by cyclin dependent kinase (CDK), Polo kinase (PLK) and the Skp, Cullin, F-boxÃ_Â_TrCP (SCFÃ_Â_TrCP) E3 ubiquitin ligase complex. His findings indicate that CDK phosphorylates NUAK1 at Ser445, triggering binding to PLK. PLK1 then subsequently phosphorylates NUAK1 at two conserved non-catalytic Ser residues (Ser476 and Ser480). This induces binding of NUAK1 to Ã_Â_TrCP, the substrate recognition subunit of the SCFÃ_Â_TrCP E3 ligase, resulting in NUAK1 becoming ubiquitylated and degraded.
Sourav also found that NUAK1 and PLK1 are reciprocally controlled in the cell cycle. In G2-M phase when PLK1 is most active, NUAK1 levels are low and vice versa in S-phase when PLK1 activity is low, NUAK1 is more highly expressed. Moreover, Sourav found that NUAK1 inhibitors (WZ4003 or HTH-01-015) suppresses proliferation by reducing the population of cells in S-phase and mitosis an effect that can be rescued by overexpression of a NUAK1 mutant in which the Ser476 and Ser480 residues are mutated to Ala to prevent ubiquitylation by SCFÃ_Â_TrCP.
Sourav also showed that consistent with a role of NUAK1 inhibiting PP1Ã_Â_MYPT1 and PP1Ã_Â_MYPT1 acting on PLK1, that subjecting cells to a treatment that induces them to detach from the matrix that they are growing on, which results in a marked phosphorylation and inhibition of PP1Ã_Â_MYPT1 by NUAK1, resulted in a significantly enhanced the T-loop phosphorylation of PLK1 at Thr210. This phosphorylation of the Thr210 induced by NUAK1 phosphorylation of MYPT1-is accompanied by an electrophoretic band-shift of PLK1 indicating that the stoichiometry of phosphorylation is significant. Importantly, this effect on PLK1 Thr210 phosphorylation following cell detachment was prevented by the NUAK1 selective WZ4003 inhibitor.
Sourav's work provides further insights into the biological regulation of the NUAK isoforms and highlights the remarkable interplay that exists between CDKs, Polo kinase, NUAK1, PP1Ã_Â_MYPT1 and SCFÃ_Â_TrCP signalling components (see Figure). To read a copy of Sourav's paper click here.
Sourav is now departing the MRC-PPU to start a postdoc in Jack Dixon's laboratory in San Diego and we wish him all the best for the future.
Only a single substrate, namely the MYPT1 subunit of the PP1Ã_Â_MYPT1 myosin phosphatase complex, has been reported. This finding was made by Anna Zagorska who previously undertook her PhD in the Alessi lab in the MRC-PPU. Anna's data suggested that NUAK1 phosphorylation of PP1Ã_Â_MYPT1 triggered binding to 14-3-3 isoforms and lead to an inhibition of phosphatase activity.
Previous work by other groups has also revealed that PP1Ã_Â_MYPT1 acts to inactivate PLK1 by dephosphorylating the T-loop Thr210 residue, thereby controlling mitosis. The ability of PLK1 to interact with PP1Ã_Â_MYPT1 is dependent upon phosphorylation of MYPT1 at Ser473 by the cyclin dependent protein kinase-2 (CDK2), which creates a docking site recognised by the Polo-box domain of PLK1. Interestingly, Ser473 lies immediately adjacent to the NUAK1 phosphorylation site (Ser472) that controls 14-3-3 binding. This therefore suggests that phosphorylation of MYPT1 by NUAK1 and 14-3-3 binding could directly interfere with the ability of PP1Ã_Â_MYPT1 to interact with and hence dephosphorylate PLK1.
Intrigued by all of this, a PhD Student in Alessi lab, Sourav Banerjee, has spent the last few years exploring links between NUAK1 and components that regulate cell cycle and PLK1. This research has lead Sourav to discover that expression of NUAK1 is controlled by cyclin dependent kinase (CDK), Polo kinase (PLK) and the Skp, Cullin, F-boxÃ_Â_TrCP (SCFÃ_Â_TrCP) E3 ubiquitin ligase complex. His findings indicate that CDK phosphorylates NUAK1 at Ser445, triggering binding to PLK. PLK1 then subsequently phosphorylates NUAK1 at two conserved non-catalytic Ser residues (Ser476 and Ser480). This induces binding of NUAK1 to Ã_Â_TrCP, the substrate recognition subunit of the SCFÃ_Â_TrCP E3 ligase, resulting in NUAK1 becoming ubiquitylated and degraded.
Sourav also found that NUAK1 and PLK1 are reciprocally controlled in the cell cycle. In G2-M phase when PLK1 is most active, NUAK1 levels are low and vice versa in S-phase when PLK1 activity is low, NUAK1 is more highly expressed. Moreover, Sourav found that NUAK1 inhibitors (WZ4003 or HTH-01-015) suppresses proliferation by reducing the population of cells in S-phase and mitosis an effect that can be rescued by overexpression of a NUAK1 mutant in which the Ser476 and Ser480 residues are mutated to Ala to prevent ubiquitylation by SCFÃ_Â_TrCP.
Sourav also showed that consistent with a role of NUAK1 inhibiting PP1Ã_Â_MYPT1 and PP1Ã_Â_MYPT1 acting on PLK1, that subjecting cells to a treatment that induces them to detach from the matrix that they are growing on, which results in a marked phosphorylation and inhibition of PP1Ã_Â_MYPT1 by NUAK1, resulted in a significantly enhanced the T-loop phosphorylation of PLK1 at Thr210. This phosphorylation of the Thr210 induced by NUAK1 phosphorylation of MYPT1-is accompanied by an electrophoretic band-shift of PLK1 indicating that the stoichiometry of phosphorylation is significant. Importantly, this effect on PLK1 Thr210 phosphorylation following cell detachment was prevented by the NUAK1 selective WZ4003 inhibitor.
Sourav's work provides further insights into the biological regulation of the NUAK isoforms and highlights the remarkable interplay that exists between CDKs, Polo kinase, NUAK1, PP1Ã_Â_MYPT1 and SCFÃ_Â_TrCP signalling components (see Figure). To read a copy of Sourav's paper click here.
Sourav is now departing the MRC-PPU to start a postdoc in Jack Dixon's laboratory in San Diego and we wish him all the best for the future.

