Ubiquitylation was discovered over 40 years ago as a mechanism that marks proteins for destruction by the proteasome. Since then, ubiquitylation has been found to regulate protein function in other ways, but the concept that proteins are the sole targets of ubiquitylation has never altered. Now, in a paper that has just been published in the EMBO Journal, a research team in the MRC Protein Phosphorylation and Ubiquitylation Unit at the University of Dundee, Scotland has found that carbohydrates can also be ubiquitylated.
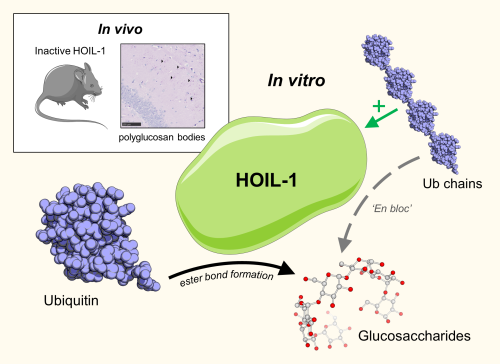
Humans deficient in a protein termed HOIL-1 (also called RBCK1) accumulate Polyglucosan Bodies (PB) in a number of organs, including the heart and brain. These insoluble, starch-like deposits, consisting largely of unbranched glycogen, precipitate in cells and cause children to develop cardiac muscle weakness, often culminating in death from heart failure.
Several years ago, Ian Kelsall, a postdoctoral research associate in Philip Cohen’s team discovered that HOIL-1 is an atypical E3 ubiquitin ligase that catalyses the formation of ester bonds between the C-terminus of ubiquitin and the hydroxyl side chains of serine and threonine residues in proteins (Kelsall et al, 2019, PNAS 116 13293-13298). The team made a knock-in mouse in which HOIL-1 was replaced by the E3 ligase-inactive HOIL-1[C458S] mutant and found that they also accumulated PB in their heart and brain, establishing that the E3 ligase activity of HOIL-1 was essential to prevent the accumulation of PB.
The team considered how the HOIL-1 E3 ligase might mediate this protective effect and wondered whether it might ubiquitylate the hydroxyl groups of glycogen directly. This led Paula Mancebo, a visiting student from Spain working with Ian Kelsall, to confirm this hypothesis. Elisha McCrory, a PhD student in the Dundee lab working in collaboration with the Steve Matthews and Yingqi Xu at the Cross Faculty NMR Centre at Imperial College London, then made the important discovery that HOIL-1 ubiquitylates the C6 hydroxyl group of glucose specifically. This hydroxyl group lies in a CH2OH side chain equivalent to the side chain of serine and is the position at which the branching of glycogen takes place. These and other findings made by Ian Kelsall revealed how HOIL-1 may recognise unbranched glycogen and led the team to propose that ubiquitylation marks unbranched glycogen for uptake into the lysosomes where it is hydrolysed by lysosomal acid alpha glucosidase, a process that has been termed glycophagy.
The team is now investigating how HOIL-1 controls glycophagy and whether sugar ubiquitylation has a wider role in human health and disease than has hitherto been realised.

