
Research conducted by De Cesare’s lab at the MRC Protein Phosphorylation and Ubiquitylation Unit, University of Dundee, Scotland, has uncovered a fascinating aspect of ubiquitin conjugating enzymes (E2s). Ubiquitin, a small protein, serves as a vital molecular tag in regulating numerous cellular functions, with E2s playing a central role in this process by facilitating the attachment of ubiquitin to target proteins (ubiquitination).
Traditionally, ubiquitination has been associated with targeting lysine side chains, referred to as canonical ubiquitination. However, in a notable discovery, Abdul Rehman et al. have identified a family of E2s (UBE2Qs) capable of attaching ubiquitin to serine and/or threonine residues, challenging conventional understanding
Arif, the first author of the study, explains: “We initially detected this unusual E2 activity via MALDI-TOF Mass Spectrometry. Subsequently, through the use of structural modeling and prediction tools, we pinpointed the key activity determinants of these E2s, enabling them to conjugate ubiquitin to serine and threonine instead of lysine.”
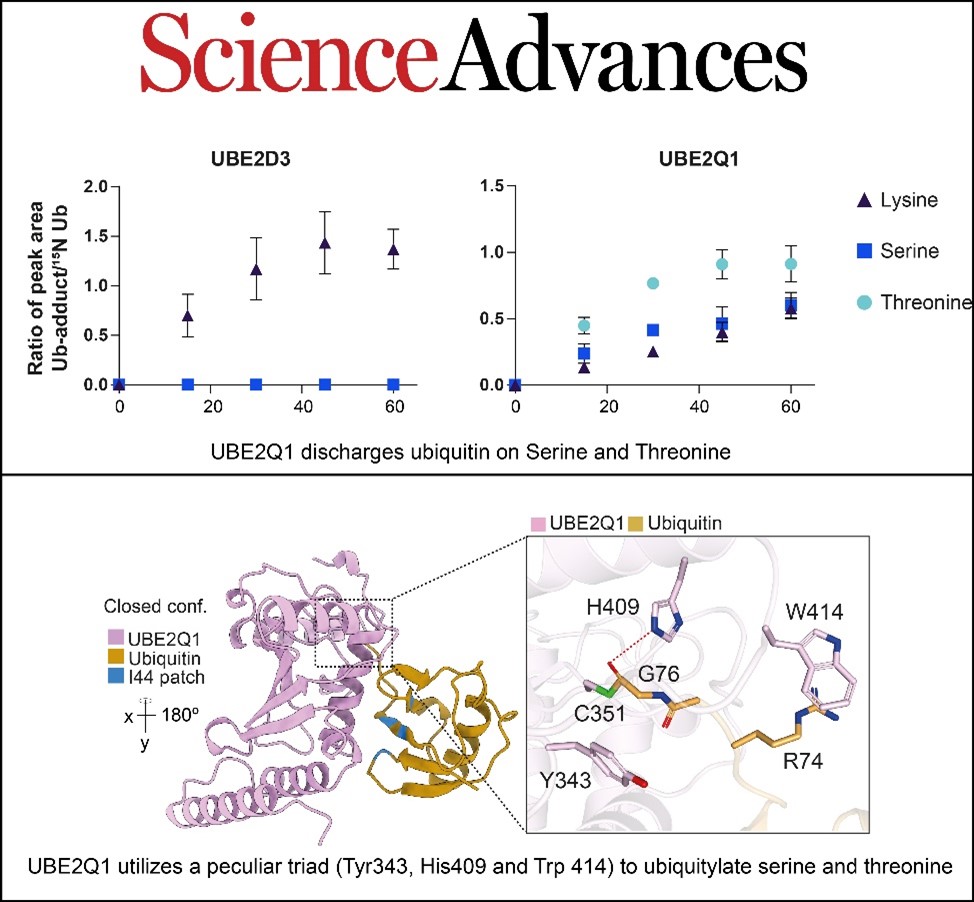
“These findings are very exciting, especially as we increasingly recognize ubiquitination's versatility as a post-translational modification," emphasizes corresponding author Dr. Virginia De Cesare. "Given the labile nature of serine and threonine ubiquitination compared to lysine ubiquitination, it's clear that we need to refine and optimize our current detection tools to effectively study it.”
This discovery enriches our understanding of ubiquitin signaling and paves the way for exciting new avenues of research in this field.
The paper was published on 27th March in Science Advances and can be found here (https://www.science.org/doi/10.1126/sciadv.adh0123)

