There has been considerable interest in understanding the function of the leucine-rich repeat kinase-2 (LRRK2) since the discovery in 2004 that autosomal dominant mutations that activate this protein kinase cause inherited Parkinson's disease. Work in the Alessi lab in the MRC PPU has been geared towards understanding how mutations in LRRK2 impair its biological functions and cause Parkinson’s.
Earlier this year in a major collaborative work with Matthias Mann’s group and researchers at GlaxoSmithKline (GSK), Merck, and The Michael J Fox Foundation for Parkinson's Research (MJFF), we discovered that LRRK2 directly phosphorylates a conserved Thr/Ser residue in the effector-binding switch-II motif of a number of Rab GTPase isoforms, including Rab10.
The data indicated that Parkinson’s causing mutations stimulate LRRK2 to directly phosphorylate a small subgroup of Rab GTPases proteins including Rab 8A and Rab10. Phosphorylation of Rab proteins by LRRK2 suppressed their interaction with GDIs as well as guanine nucleotide exchange factors that are required for membrane delivery, recycling and activation.
This led to the hypothesis that Parkinson’s mutations in LRRK2 result in inappropriate phosphorylation and inhibition of a sub group of Rab GTPases and this might impair vesicular trafficking and processes such as autophagy.
An important next goal of the research was to develop a robust method to rapidly assess LRRK2 phosphorylation of endogenous Rab isoforms in samples where material may be limiting without the need to use state of the art mass spectrometry to assess Rab protein phosphorylation.
To address this question Genta Ito, a postdoc in the Alessi lab collaboration with other researchers from the University of Dundee, MJFF, GSK, and the University of Hong Kong, elaborated a new procedure exploiting an agent (1,3-bis[bis(pyridin-2-ylmethyl) amino]propan-2-olato dizinc(II) complex) commonly referred to as “Phos-tag” that binds to phosphate ions with very higher affinity.
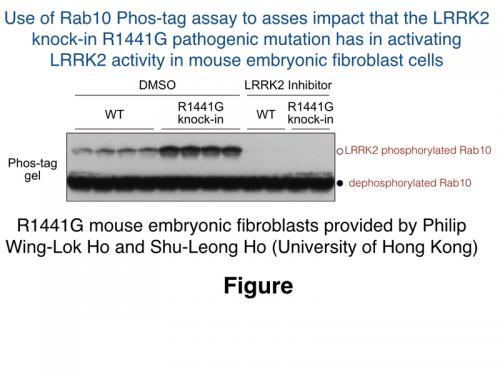
Genta found that when polymerised into SDS-polyacrylamide gels the Phos-tag reagent dramatically retarded electrophoretic mobility of LRRK2 phosphorylated Rab10, resulting in substantial mobility shifts (see figure). Using this approach, Genta was able to demonstrate that ablation of LRRK2 catalytic activity in a novel, kinase inactive LRRK2[D2017A] knock-in mouse model developed by Alastair Reith’s group at GSK, blocked Rab10 phosphorylation in mouse embryonic fibroblasts (MEFs) as well as lung, demonstrating that LRRK2 is indeed the major Rab10 kinase in these cells and tissue.
Genta also established that the Phos-tag assay can be used to monitor the impact of LRRK2 inhibitors, as well as pathogenic knock-in mutations (G2019S [kindly provided to us by GlaxoSmithKline] and R1441G [provided to us by Philip Wing-Lok Ho and Shu-Leong Ho, University of Hong Kong) on Rab10 phosphorylation (see Fig). Interestingly, Genta found that treatment of cells with LRRK2 inhibitors induced almost complete dephosphorylation of LRRK2 phosphorylated Rab10 with 1 min-indicating that the phosphatase activity acting on Rab10 must be very active in cells.
We hope that the Rab10 Phos-tag assay will aide with the assessment LRRK2 signalling pathway activity in cells and to establish the impact that inhibitors, mutations and other factors have. The prediction is that elevation of LRRK2 activity leads to Parkinson’s disease and the expectation is that if a sub-group of patients can be identified with elevated LRRK2 activity, it would be important to explore whether these individuals might benefit most from LRRK2 inhibitors that are being developed.
Therefore, a major aim of our future work will be to investigate whether this Phos-tag technology could be exploited to assess LRRK2 activity in Parkinson’s patients.
Also congratulations to Gento Ito for securing a group leader position at the University of Tokyo (Laboratory of Neuropathology and Neuroscience, Graduate School of Pharmaceutical Sciences). Genta will continue working on better understanding LRRK2 and how this is linked to Parkinson’s.
To read the paper describing these findings click here and for the press release accompanying this paper click here.

