The serum- and glucocorticoid-regulated kinase-3 (SGK3) contribute resistance to cancer therapies targeting the PI3K pathway. Recent work has shown that the SGK3 isoform is upregulated in breast cancer cells treated with PI3K or Akt inhibitors and recruited and activated at endosomes, through its phox homology domain binding to PtdIns(3)P.
SGK3 is homologous to Akt, display overlapping specificity and phosphorylate several substrates at the same residues, such as TSC2 to promote tumor growth by switching on the mTORC1 pathway.
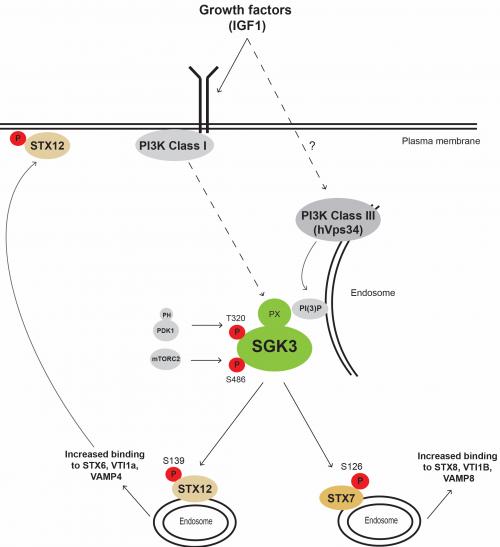
SGK3 possesses an N-terminal PtdIns(3)P-binding PX (Phox homology) domain. This targets SGK3 to endosomal membranes, where PtdIns(3)P is generated by the Class III PI3K family member, known as hVps34.
Nazma Malik (ex-MRC PPU PhD student, now working in Reuben Shaw’s laboratory, Salk, San Diego) and Athanasios Karapetsas (postdoc in the Alessi lab) working with Matthias Trost (now University of Newcastle) and Raja Nirujogi, set out to study whether selective substrates for SGK3 that are not phosphorylated by Akt exist. Such substrates would be predicted to be localized at endosomes.
Genetic and pharmacological phosphoproteomic screens were undertaken that resulted in around 40 potential novel SGK3 substrates being identified. These included 4 endosomal proteins STX7 (Ser126) and STX12 (Ser139), RFIP4 (Ser527) and WDR44 (Ser346) that were efficiently phosphorylated in vitro by SGK3 at the sites identified in vivo.
In contrast to other SGK3 substrates that have been characterized, these proteins were poorly phosphorylated by Akt. Nazma and Thanos demonstrated that these substrates were inefficiently phosphorylated by Akt, as they possessed an n+1 residue from the phosphorylation site (Gly, Ser, Arg) that is highly unfavorable for Akt phosphorylation.
Thanos demonstrated that stimulation of HEK293 cells with IGF1 to activate SGK3, promoted phosphorylation of a significant fraction of endogenous STX7 and STX12, in a manner that was blocked by knock-out of SGK3 or treatment with a pan SGK inhibitor (14H).
Nazma also generated a phospho-specific antibody that can readily recognize Ser139 SGK3 phosphorylated form of STX12 in vivo, that could be utilized as a future biomarker for SGK3 activity in vivo.
Finally, Thanos working with Alan Prescott was able to demonstrate that phosphorylation of STX12 enhanced interaction with the VAMP4/VTI1A/STX6 containing SNARE complex and promoted plasma membrane localization.
These findings reveal novel substrates for SGK3 and suggest a mechanism by which STX7 and STX12 SNARE complexes are regulated by SGK3. They reveal new biomarkers for monitoring SGK3 pathway activity.
To read the paper describing this work click here.

