Mutations that activate the Leucine-rich repeat kinase 2 (LRRK2) protein kinase, predispose to Parkinson’s disease, suggesting that LRRK2 inhibitors might have therapeutic benefit. Our previous work revealed that LRRK2 phosphorylates a group of 3 Rab proteins (Rab8A, Rab10 and Rab12) at a highly conserved Thr/Ser residue at the centre of the effector binding motif.
All LRRK2 pathogenic mutations tested, including those located in the kinase domain (G2019S) or GTPase/COR domain (R1441G or Y1699C) significantly increase Rab8, Rab10 and Rab12 protein phosphorylation in vivo.
Further analysis revealed that the LRRK2 phosphorylation site is conserved in 50 of the 70 known Rab protein. This suggested that LRRK2 had the potential to phosphorylate Rab beyond Rab8A, Rab10 and Rab12.
In a major collaboration with the Mann lab, we cloned and expressed all 50 Rab proteins in a HEK293 cells expression system and evaluated whether LRRK2 could phosphorylate these Rab proteins either using the Phos-tag approach (experiments done by Federico Diez, MRC PPU) or by mass spectrometry (experiments done by Martin Steger in the Mann lab).
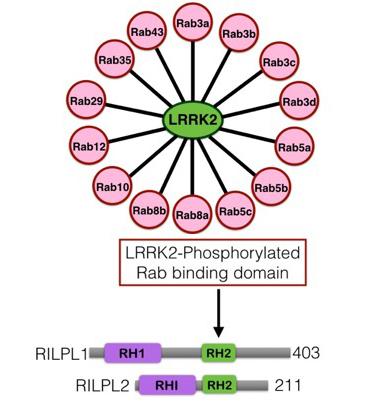
Both approaches strikingly revealed that LRRK2 phosphorylated 14 Rab proteins (Rab3A, Rab3B, Rab3C, Rab3D, Rab5A, Rab5B, Rab5C, Rab8A, Rab8B, Rab10, Rab12, Rab29, Rab35 and Rab43) all at the equivalent site apart from Rab29 that is phosphorylated at two adjacent residues (Thr71, Ser72).
Interestingly, the Mann lab found that 20 other Rab proteins were also phosphorylated at the equivalent residue by a protein kinases other than LRRK2. Thus, at least 34 of the 70 Rab proteins are phosphorylated within the effector-binding Switch-II motif. This is likely to represent a major mechanism by which the function of Rab proteins are controlled.
Marin Steger as well as MRC PPU postdocs Pawel Lis and Raja Nirujogi, immunoprecipitated endogenous Rab proteins from wild type and LRRK2 pathogenic R1441C knock-in cells. Immunoprecipitates were analysed by mass spectrometry as well as with phospho-specific antibodies. These studies revealed that 10 Rab proteins (Rab3A, Rab3B, Rab3C, Rab3D, Rab8A, Rab8B, Rab10, Rab12, Rab35 and Rab43) are likely phosphorylated by endogenous LRRK2 at least in mouse embryonic fibroblast and HEK293 cells.
Particularly useful for these types of experiments will be the MJFF-pRAB8 antibody (see Lis et al Biochemical J 2017 paper), that immunoprecipitates 10 out of the 14 LRRK2 phosphorylated Rab proteins
The stoichiometry of LRRK2 mediated phosphorylation of endogenous Rab proteins in cells is very low, with only 1-3% of the protein being phosphorylated. We wondered whether LRRK2 mediated phosphorylation of Rab proteins rather than serving to inhibit the interaction with known effectors, would work instead to enable Rab to bind novel receptor proteins.
This motivated both Federico Diez and Martin Steger to independently undertake mass spectrometry screens to identify sets of proteins that interact selectively with LRRK2 phosphorylated Rab8A and Rab10. This resulted in Federico Diez first identifying RILPL1 as a protein that bound LRRK2 phosphorylated Rab8 and in subsequent independent studies Martin Steger identified a related protein termed RILPL2. RILPL1 and RILPL2 are largely unstudied proteins implicated in one study as possibly regulating regulate ciliary membrane content.
Further studies confirm that in cells that RILPL1 and RILPL2 bind to LRRK2 phosphorylated Rab8A, Rab10 and Rab12 through a likely coiled-coiled domain known as RH2. We identified 3 very conserved Arg/Lys residues found in the RH2 domain of both RILPL1 and RILPL2 that Federico Diez demonstrated that when these residues were mutated to non-basic residues prevented interaction with LRRK2 phosphorylated Rab8A, Rab10 and Rab12.
In further work undertaken by the Mann and Pfeffer labs, it was discovered that induction of primary cilia formation by serum starvation was inhibited by the pathogenic LRRK2-R1441G knock-in mutation in a manner that could be rescued by inhibiting LRRK2. This is first time, a connection between LRRK2 and cilia formation has been demonstrated.
In future research, it will be important to focus on improved reagents and technologies to better study the 14 LRRK2 Rab substrates and to define what roles these play in different cells including dopaminergic neurons. Why LRRK2 preferentially phosphorylates these 14 Rab proteins in vivo and not others is also not known and would be important to understand.
A crucial but hard to answer question, is which of the 14 Rab proteins(s) targeted by LRRK2 is of most relevance to understanding how LRRK2 is linked to Parkinson’s disease. It will also be extremely important to determine the roles that RILPL1 and RILPL2 play in controlling downstream biology regulated by LRRK2. Whether RILPL1 and RILPL2 is linked to Parkinson’s disease will be a major question for future work. We are very grateful to the support of the Michael J Fox Foundation for this research.
To read a copy of the paper that describes this work click here.

