The Wnt signalling pathway plays fundamental roles in shaping developing embryos and controlling cell fate in adults. Mutations that cause slight alterations in Wnt signalling are associated with developmental defects as well as a myriad of diseases, such as cancer.
Research scientists from the University of Dundee’s Medical Research Council Protein Phosphorylation and Ubiquitylation Unit and the Francis Crick Institute have uncovered a crucial role for the protein PAWS1 (aka FAM83G) in controlling the Wnt signalling pathway. The same researchers had earlier made the discovery of PAWS1 as a protein associated with Smad1, which is a transcription factor that mediates Bone Morphogenetic Protein signalling.
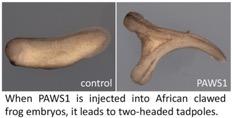
The activation of Wnt signalling in frog embryos is known to cause a two-headed tadpole, an event that results from a complete duplication of the embryonic axis. The researchers found that injection of PAWS1 into frog embryos resulted in two-head tadpoles, thus implicating PAWS1 in the control of Wnt signalling. When the researchers removed PAWS1 from a human osteosarcoma cell line, they found that Wnt signalling was inhibited. To further explore the molecular mechanisms through which PAWS1 controls Wnt signalling, the researchers discovered that PAWS1 physically associates with Casein Kinase 1 alpha (CK1a), which is an enzyme that is known to regulate Wnt signalling. They went on to map the determinants of PAWS1:CK1a interaction and subsequently demonstrated that PAWS1 mutations incapable of associating with CK1a not only fail to cause two-headed tadpoles but are also unable to activate Wnt signalling.
The findings are published in the current issue of EMBO Reports. Polyxeni Bozatzi (PhD student, Sapkota Lab) and Dr. Kevin Dingwell (Smith Lab) are joint lead authors of this study. Additionally, Kevin Wu, Timothy Cummins, Luke Hutchinson, Janis Vogt, Nicola Wood, Thomas Macartney, Joby Varghese, Robert Gourlay and David Campbell (from MRC PPU/DSTT, Dundee) and Fay Cooper (from Crick) also contributed to the study.
Dr. Gopal Sapkota (Dundee), who led this research jointly with Professor Sir Jim Smith (Crick), said “our uncovering of PAWS1 as a crucial regulator of CK1a in Wnt signalling represents a significant leap in our understanding of this important signalling pathway. CK1a has been known as an important player in Wnt signalling for two decades but its regulation had remained a mystery until now.”
Professor Smith said, “Although the precise function of PAWS1 were poorly understood, PAWS1 mutations are known to cause palmoplantar hyperkeratosis, a disease in which there is excessive skin cell growth on the soles and palms and can affect normal hair growth leading to conditions such as alopecia. Wnt signalling is known to play crucial roles in the maintenance of skin tissue and hair development. Our findings that PAWS1 is important player in Wnt signalling now offer an opportunity to establish whether the pathogenic PAWS1 mutations impact Wnt signalling to give rise to this disease.”
“Abnormal Wnt signalling is associated with many cancers, particularly colorectal cancers. Understanding how PAWS1 regulates Wnt signalling may therefore offer new opportunities and targets for potential interventions,” said Dr. Sapkota.
Prof. Smith added, “This research is an outcome of a long-standing collaboration between the Sapkota (Dundee) and Smith (Crick) labs. This work represents a wonderful example of how collaboration facilitates key scientific discoveries.”
[Jim Smith is Director of Science at Wellcome Trust, but made his contribution to this paper as part of his role as a visiting Group Leader at the Crick. Wellcome Trust had no role in the writing or decision to submit this paper for publication, except for the involvement of Jim Smith as a contributing author.]

