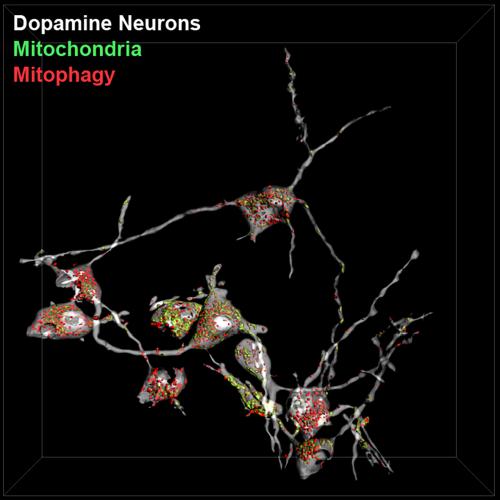
Mitophagy is the autophagic removal of damaged or impaired mitochondria. A new study published in Cell Metabolism from Ian Ganley and colleagues, shows for the first time that dopaminergic neurons within the substantia nigra undergo a striking amount of mitophagy. This is important because it is this population of neurons that degenerate in Parkinson’s Disease (PD) and impaired mitophagy has been implicated in this pathology. Damaged mitochondria can be very harmful to the cell and can lead to cell death if not dealt with. Consequently, given the large amount of mitophagy that is now evident in these neuronal cells, it is reasonable to assume that interfering with this process would be very detrimental to their health. Thus, this pathway could be a viable PD therapeutic target.
The authors used the lab’s recently developed mito-QC mouse that expresses a fluorescent mitophagy reporter and were able to show that mitophagy was prevalent not just in neurons, but also in other tissues of high metabolic demand, such as muscle, retina and pancreas. How then is this mitophagy regulated and can it be exploited for disease therapy? Activation of the protein kinase PINK1, which is mutated in some forms of hereditary PD, has been shown to play a key role in driving mitophagy in cell lines, but whether this is also true in adult dopaminergic neurons, which cannot be cultured in vitro, was unknown. The first author of this study, Dr. Tom McWilliams, was able to show that surprisingly, loss of PINK1 did not alter the basal rate of mitophagy in PD-relevant dopaminergic neurons, or other tissues. Thus, the conundrum of PINK1 function in vivo is far from solved and if PINK1 is not regulating this mitophagy, then what is? The race is on to solve these questions.
This work was funded by the Medical Research Council and the Michael J. Fox Foundation for Parkinson’s Research and is published in the journal Cell Metabolism: “Basal Mitophagy Occurs Independently of PINK1 in Mouse Tissues of High Metabolic Demand” Thomas G. McWilliams, Alan R. Prescott, Lambert Montava-Garriga, Graeme Ball, François Singh, Erica Barini, Miratul M.K. Muqit, Simon P. Brooks, Ian G. Ganley.

