Alberto Vitari makes fascinating discovery
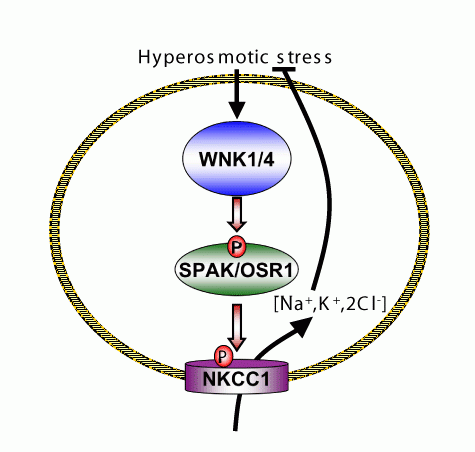
There was much excitement in 2001 when elegant clinical investigations undertaken by Richard Lifton's research group at the Yale University School of Medicine, New Haven reported that the WNK1 and WNK4 protein kinases, were mutated in patients that suffered from a severe inherited hypertension syndrome called 'Gordon Syndrome'. As the function of WNK kinases was unknown, Alberto Vitari, a PhD student in the MRC Unit, decided to undertake the challenge of identifying physiological substrates for the WNK1 and WNK4 enzymes, as this might provide new clues as to how hypertension is regulated at the molecular level. Alberto made the interesting discovery that WNK1 and WNK4 interacted with high affinity with two other closely related protein kinases in cells that were termed SPAK and OSR1. Alberto then found that SPAK and OSR1 were efficiently phosphorylated by WNK1 and WNK4 and most interestingly, the activity of SPAK and OSR1 was hugely enhanced following phosphorylation with WNK1 and WNK4. Fascinatingly, SPAK and OSR1 were previously reported to be activated in cells in response to osmotic stress such as high salt and phosphorylate and regulate the activity of ion transporters such as NKCC1. It had also been previously shown that treatment of cells with high salt concentration activated WNK1. Putting this data together with Alberto's results, suggest that osmotic stress control the activity of ion transporters through the WNK-SPAK/OSR1 pathway (see Fig). It is possible that mutations in WNK1 and WNK4 genes in humans disrupt this pathway, thereby affecting ion transporter activity in organs such as the kidney, which could account for the development of hypertension in subjects with these mutations.
Click here to access Alberto's paper.
Click here to access Alberto's paper.

