Characterisation of VPS34 inhibitor reveals PtdIns 3-P binding SGK3 protein kinase is downstream target of Class III PI-3 kinase
Phosphoinositide 3-kinases (PI3K) phosphorylate the 3'-position hydroxyl of the D-myo-inositol head group of phosphatidylinositol (PtdIns) to generate 3-phosphoinositides that play critical in orchestrating a wide range of biological responses including controlling cell growth, proliferation and intracellular trafficking.
There are three different Classes of PI3Ks termed Class I, Class II and Class III. The most studied enzymes comprise the four members of Class I PI3Ks (p110alpha, p110beta p110gamma and p110delta) family. These phosphorylate PtdIns(4,5)P2 at the plasma membrane to generate the signalling second messenger PtdIns(3,4,5)P3 in response to agonists that trigger activation of growth factors, Ras or G protein coupled receptors.
Excitingly, the first PI3K inhibitor termed Idelalisib, developed by Gilead Sciences that targets the p110 delta isoform, has recently received FDA approval. Idelalisib is designed to be used combination with rituximab for chronic lymphocytic leukaemia and predicted to generate 1.3 billion dollars in sales. The expectations are that this drug and other PI3K inhibitors being developed will also be useful in treating solid tumours.
In contrast, the roles of the 4 isoforms of the Class II and single isoform of Class III PI3K isoform (also termed Vps34), in comparison to Class I PI3Ks, are much less well understood. Their function seem to be to phosphorylate PtdIns in order to generate PtdIns(3)P. Evidence to date indicates that Vps34 plays an important role in controlling vesicular protein sorting.
Ruzica Bago, a postdoc in Dario Alessi's lab, in collaboration with a number of other researchers including two Phd Students [Nazma Malik (Alessi lab) and Michael Munson (Ganley lab)] as well as AstraZeneca researchers (Richard Ward and Darren Cross), was interested in characterising a pharmacological inhibitor that would permit the the role of the Class III PI3Ks to be better investigated. Ruzica was also curious to determine whether VPS34 played a role in regulating the activity of the only protein kinase in the cell known to interact with PtdIns(3)P, namely the serum and glucocorticoid protein kinase-3 (SGK3).
To undertake this project Ruzica first painstakingly analysed the potency and specificity of a number of potential VPS34 inhibitors that have been reported in the patent literature. This led to the identification of a compound that we termed VPS34-IN1, which inhibited recombinant Vps34 with nanomolar potency, but did but does not significantly inhibit the activity of 340 protein kinases or 25 lipid kinases tested that include all isoforms of Class I as well as Class II PI3K. Ruzica was able to demonstrate that VPS34-IN1 when added to cells, rapidly reduced endosomal PtdIns(3)P levels within 1 min of drug treatment.
Ruzica then established that mutations in SGK3 that prevented PtdIns(3)P-binding also ablated SGK3 kinase activity by suppressing phosphorylation of the T-loop (PDK1 site) and hydrophobic motif (mTOR site) residues. Ruzica found that VPS34-IN1 in cells induced a rapid ~50-60% loss of SGK3 phosphorylation. Furthermore, Ruzica also found that Class I PI3K inhibitors (GDC-0941, BKM120) that do not inhibit Vps34, suppressed SGK3 activity by ~40%. Combining VPS34-IN1 and GDC-0941 reduced SGK3 activity ~80-90%.
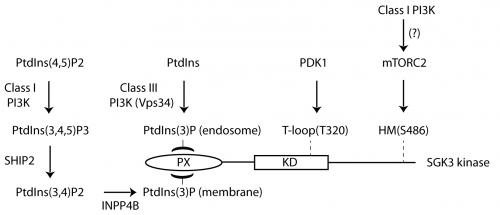
We have interpreted these data to suggest that SGK3 phosphorylation and hence activity is controlled by two pools of PtdIns(3)P. The first is produced through phosphorylation of PtdIns by Vps34 at the endosome. The second is due to the conversion of Class I PI3K product, PtdIns(3,4,5)P3 to PtdIns(3)P, via the sequential actions of the PtdIns 5-phosphatases (SHIP1/2) and PtdIns 4-phosphatase (INPP4B) (See Figure).
We believe that VPS34-IN1 will be a useful probe to delineate physiological roles of the Vps34.
We propose that monitoring SGK3 phosphorylation and activity could be employed as a biomarker of Vps34 activity, in an analogous manner by which Akt is used to probe cellular Class I PI3K activity.
Finally, it would be interesting to explore whether combining Class I (e.g. GDC-0941) and Class III (e.g. VPS34-IN1) PI3K inhibitors, could be used as a strategy to better analyse the roles and regulation of the elusive Class II PI3K.
To read a copy of Ruzica's paper that has just been published click here.
There are three different Classes of PI3Ks termed Class I, Class II and Class III. The most studied enzymes comprise the four members of Class I PI3Ks (p110alpha, p110beta p110gamma and p110delta) family. These phosphorylate PtdIns(4,5)P2 at the plasma membrane to generate the signalling second messenger PtdIns(3,4,5)P3 in response to agonists that trigger activation of growth factors, Ras or G protein coupled receptors.
Excitingly, the first PI3K inhibitor termed Idelalisib, developed by Gilead Sciences that targets the p110 delta isoform, has recently received FDA approval. Idelalisib is designed to be used combination with rituximab for chronic lymphocytic leukaemia and predicted to generate 1.3 billion dollars in sales. The expectations are that this drug and other PI3K inhibitors being developed will also be useful in treating solid tumours.
In contrast, the roles of the 4 isoforms of the Class II and single isoform of Class III PI3K isoform (also termed Vps34), in comparison to Class I PI3Ks, are much less well understood. Their function seem to be to phosphorylate PtdIns in order to generate PtdIns(3)P. Evidence to date indicates that Vps34 plays an important role in controlling vesicular protein sorting.
Ruzica Bago, a postdoc in Dario Alessi's lab, in collaboration with a number of other researchers including two Phd Students [Nazma Malik (Alessi lab) and Michael Munson (Ganley lab)] as well as AstraZeneca researchers (Richard Ward and Darren Cross), was interested in characterising a pharmacological inhibitor that would permit the the role of the Class III PI3Ks to be better investigated. Ruzica was also curious to determine whether VPS34 played a role in regulating the activity of the only protein kinase in the cell known to interact with PtdIns(3)P, namely the serum and glucocorticoid protein kinase-3 (SGK3).
To undertake this project Ruzica first painstakingly analysed the potency and specificity of a number of potential VPS34 inhibitors that have been reported in the patent literature. This led to the identification of a compound that we termed VPS34-IN1, which inhibited recombinant Vps34 with nanomolar potency, but did but does not significantly inhibit the activity of 340 protein kinases or 25 lipid kinases tested that include all isoforms of Class I as well as Class II PI3K. Ruzica was able to demonstrate that VPS34-IN1 when added to cells, rapidly reduced endosomal PtdIns(3)P levels within 1 min of drug treatment.
Ruzica then established that mutations in SGK3 that prevented PtdIns(3)P-binding also ablated SGK3 kinase activity by suppressing phosphorylation of the T-loop (PDK1 site) and hydrophobic motif (mTOR site) residues. Ruzica found that VPS34-IN1 in cells induced a rapid ~50-60% loss of SGK3 phosphorylation. Furthermore, Ruzica also found that Class I PI3K inhibitors (GDC-0941, BKM120) that do not inhibit Vps34, suppressed SGK3 activity by ~40%. Combining VPS34-IN1 and GDC-0941 reduced SGK3 activity ~80-90%.
We have interpreted these data to suggest that SGK3 phosphorylation and hence activity is controlled by two pools of PtdIns(3)P. The first is produced through phosphorylation of PtdIns by Vps34 at the endosome. The second is due to the conversion of Class I PI3K product, PtdIns(3,4,5)P3 to PtdIns(3)P, via the sequential actions of the PtdIns 5-phosphatases (SHIP1/2) and PtdIns 4-phosphatase (INPP4B) (See Figure).
We believe that VPS34-IN1 will be a useful probe to delineate physiological roles of the Vps34.
We propose that monitoring SGK3 phosphorylation and activity could be employed as a biomarker of Vps34 activity, in an analogous manner by which Akt is used to probe cellular Class I PI3K activity.
Finally, it would be interesting to explore whether combining Class I (e.g. GDC-0941) and Class III (e.g. VPS34-IN1) PI3K inhibitors, could be used as a strategy to better analyse the roles and regulation of the elusive Class II PI3K.
To read a copy of Ruzica's paper that has just been published click here.

