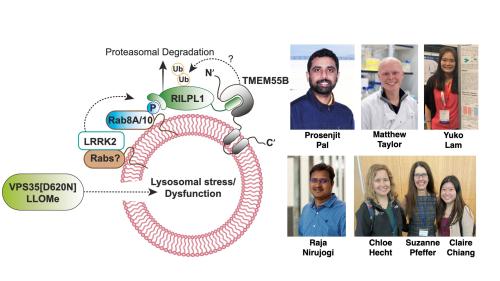
Parkinson’s VPS35[D620N] mutation induces LRRK2 mediated lysosomal association of RILPL1 and TMEM55B
We previously observed that the Parkinson’s VPS35[D620N] mutation markedly enhances LRRK2 kinase activation by an unknown mechanism.
In recent work that has just been published, postdoctoral researchers Prosenjit Pal and Yuko Lam and PhD student Matthew Taylor, together with support from Raja Nirujogi as well as with Chloe Hecht and Claire Chiang in Suzanne Pfeffer’s lab at the University of Stanford, as part of our Aligning Science Across Parkinson’s collaboration, set out to uncover how the VPS35[D620N] mutation induces activation of the LRRK2 pathway.
Experiments revealed that the VPS35[D620N] mutation triggers recruitment of LRRK2 to the lysosome where it is activated, and phosphorylates its Rab protein substrates such as Rab10, which in turn associate with the phosphoRab effector protein RILPL1.
Further experiments revealed that the LRRK2 phosphorylated Rab10:RILPL1 complex binds to a poorly studied lysosomal integral membrane protein termed TMEM55B.
To validate this model, we demonstrated that the recruitment of RILPL1 to lysosome and TMEM55B was controlled through LRRK2 activity and blocked following inhibition of LRRK2.
We identified highly conserved regions of RILPL1 and TMEM55B that interact and designed mutations that block binding.
Our proposed model, that still requires experimental validation, is that interaction of LRRK2 phosphorylated Rab proteins with RILPL1 at the lysosomal membrane, induces a conformational change in RILPL1, enabling it to bind TMEM55B.
We also observed, that in mouse fibroblasts, brain, and lung, the VPS35 [D620N] mutation markedly reduces RILPL1 levels in whole cell extracts, in a manner that was reversed by LRRK2 inhibition. This effect is dependent on proteosome activity and RILPL1 levels are increased following knock-out of TMEM55B.
This data suggested that the LRRK2 pathway may regulate an E3 ligase that acts upon RILPL1 via TMEM55B.
The lysosomotropic agent LLOMe, also induced LRRK2 kinase mediated association of RILPL1 to the lysosome, but to a lower extent than the D620N mutation.
An important unanswered question is how does the VPS35[D620N] mutation recruit LRRK2 to the lysosome. Our work shows that the Parkinson’s VPS35[D620N] mutation has significant lysosomal impact, where it alters the expression of over 200 lysosomal proteins.
Our model is therefore VPS35[D620N] triggers a form of lysosomal dysfunction that stimulates an as yet unknown pathway, recruiting LRRK2 to the lysosome. Deciphering this pathway is a key future research priority.
Our study uncovers a new pathway through which dysfunctional lysosomes resulting from the VPS35[D620N] mutation recruit and activate LRRK2 on the lysosomal surface, driving assembly of the RILPL1-TMEM55B complex.
Our data reveal that TMEM55B is a novel downstream component of the LRRK2 Parkinson’s signalling pathway.
Further work is needed to explore the roles that TMEM55B plays in Parkinson’s related lysosomal dysfunction and whether this line of research could also lead to new biomarkers of Parkinson’s relevant lysosomal dysfunction.
Finally, this is our first manuscript where all of the mass spectrometry data was analysed and made freely available using our new CURTAIN tool (https://www.biorxiv.org/content/10.1101/2023.07.25.550405v1). This is designed to enable the non-expert user to peruse, analyse and share mass spectrometry data.
To read the manuscript describing this research please click here

